The CD300e molecule in mice is an immune-activating receptor
- PMID: 29358324
- PMCID: PMC5846139
- DOI: 10.1074/jbc.RA117.000696
The CD300e molecule in mice is an immune-activating receptor
Abstract
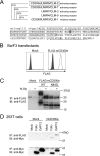
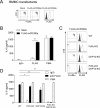
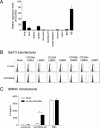
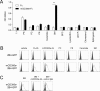
CD300 molecules (CD300s) belong to paired activating and inhibitory receptor families, which mediate immune responses. Human CD300e (hCD300e) is expressed in monocytes and myeloid dendritic cells and transmits an immune-activating signal by interacting with DNAX-activating protein 12 (DAP12). However, the CD300e ortholog in mice (mCD300e) is poorly characterized. Here, we found that mCD300e is also an immune-activating receptor. We found that mCD300e engagement triggers cytokine production in mCD300e-transduced bone marrow-derived mast cells (BMMCs). Loss of DAP12 and another signaling protein, FcRγ, did not affect surface expression of transduced mCD300e, but abrogated mCD300e-mediated cytokine production in the BMMCs. Co-immunoprecipitation experiments revealed that mCD300e physically interacts with both FcRγ and DAP12, suggesting that mCD300e delivers an activating signal via these two proteins. Binding and reporter assays with the mCD300e extracellular domain identified sphingomyelin as a ligand of both mCD300e and hCD300e. Notably, the binding of sphingomyelin to mCD300e stimulated cytokine production in the transduced BMMCs in an FcRγ- and DAP12-dependent manner. Flow cytometric analysis with an mCD300e-specific Ab disclosed that mCD300e expression is highly restricted to CD115+Ly-6Clow/int peripheral blood monocytes, corresponding to CD14dim/+CD16+ human nonclassical and intermediate monocytes. Loss of FcRγ or DAP12 lowered the surface expression of endogenous mCD300e in the CD115+Ly-6Clow/int monocytes. Stimulation with sphingomyelin failed to activate the CD115+Ly-6Clow/int mouse monocytes, but induced hCD300e-mediated cytokine production in the CD14dimCD16+ human monocytes. Taken together, these observations indicate that mCD300e recognizes sphingomyelin and thereby regulates nonclassical and intermediate monocyte functions through FcRγ and DAP12.
Keywords: cell signaling; cell surface receptor; cellular immune response; immunology; monocyte.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Yotsumoto K., Okoshi Y., Shibuya K., Yamazaki S., Tahara-Hanaoka S., Honda S., Osawa M., Kuroiwa A., Matsuda Y., Tenen D. G., Iwama A., Nakauchi H., and Shibuya A. (2003) Paired activating and inhibitory immunoglobulin-like receptors, MAIR-I and MAIR-II, regulate mast cell and macrophage activation. J. Exp. Med. 198, 223–233 10.1084/jem.20021825 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

