Cortical Connections Position Primate Area 25 as a Keystone for Interoception, Emotion, and Memory
- PMID: 29358365
- PMCID: PMC5815452
- DOI: 10.1523/JNEUROSCI.2363-17.2017
Cortical Connections Position Primate Area 25 as a Keystone for Interoception, Emotion, and Memory
Abstract
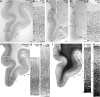
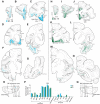
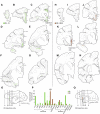
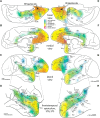
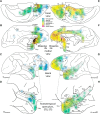
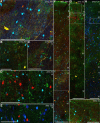
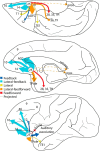
The structural and functional integrity of subgenual cingulate area 25 (A25) is crucial for emotional expression and equilibrium. A25 has a key role in affective networks, and its disruption has been linked to mood disorders, but its cortical connections have yet to be systematically or fully studied. Using neural tracers in rhesus monkeys, we found that A25 was densely connected with other ventromedial and posterior orbitofrontal areas associated with emotions and homeostasis. A moderate pathway linked A25 with frontopolar area 10, an area associated with complex cognition, which may regulate emotions and dampen negative affect. Beyond the frontal lobe, A25 was connected with auditory association areas and memory-related medial temporal cortices, and with the interoceptive-related anterior insula. A25 mostly targeted the superficial cortical layers of other areas, where broadly dispersed terminations comingled with modulatory inhibitory or disinhibitory microsystems, suggesting a dominant excitatory effect. The architecture and connections suggest that A25 is the consummate feedback system in the PFC. Conversely, in the entorhinal cortex, A25 pathways terminated in the middle-deep layers amid a strong local inhibitory microenvironment, suggesting gating of hippocampal output to other cortices and memory storage. The graded cortical architecture and associated laminar patterns of connections suggest how areas, layers, and functionally distinct classes of inhibitory neurons can be recruited dynamically to meet task demands. The complement of cortical connections of A25 with areas associated with memory, emotion, and somatic homeostasis provide the circuit basis to understand its vulnerability in psychiatric and neurologic disorders.SIGNIFICANCE STATEMENT Integrity of the prefrontal subgenual cingulate cortex is crucial for healthy emotional function. Subgenual area 25 (A25) is mostly linked with other prefrontal areas associated with emotion in a dense network positioned to recruit large fields of cortex. In healthy states, A25 is associated with internal states, autonomic function, and transient negative affect. Constant hyperactivity in A25 is a biomarker for depression in humans and may trigger extensive activation in its dominant connections with areas associated with emotions and internal balance. A pathway between A25 and frontopolar area 10 may provide a critical link to regulate emotions and dampen persistent negative affect, which may be explored for therapeutic intervention in depression.
Keywords: connectome; cortical systematic variation; inhibitory neurons; mood disorders; subgenual cingulate; tract-tracing.
Copyright © 2018 the authors 0270-6474/18/381677-22$15.00/0.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
