Centipedes subdue giant prey by blocking KCNQ channels
- PMID: 29358396
- PMCID: PMC5816164
- DOI: 10.1073/pnas.1714760115
Centipedes subdue giant prey by blocking KCNQ channels
Abstract
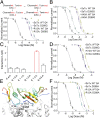
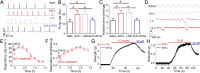
Centipedes can subdue giant prey by using venom, which is metabolically expensive to synthesize and thus used frugally through efficiently disrupting essential physiological systems. Here, we show that a centipede (Scolopendra subspinipes mutilans, ∼3 g) can subdue a mouse (∼45 g) within 30 seconds. We found that this observation is largely due to a peptide toxin in the venom, SsTx, and further established that SsTx blocks KCNQ potassium channels to exert the lethal toxicity. We also demonstrated that a KCNQ opener, retigabine, neutralizes the toxicity of a centipede's venom. The study indicates that centipedes' venom has evolved to simultaneously disrupt cardiovascular, respiratory, muscular, and nervous systems by targeting the broadly distributed KCNQ channels, thus providing a therapeutic strategy for centipede envenomation.
Keywords: KCNQ; SsTx; centipede; toxicity.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Balgooyen TC. Behaviour and ecology of the American kestrel (Falco sparverius L.) in the Sierra Nevada of California. Univ Calif Publ Zool. 1976;103:1–84.
-
- Griffiths D. Foraging costs and relative prey size. Am Nat. 1980;116:743–752.
-
- Dufton MJ. Venomous mammals. Pharmacol Ther. 1992;53:199–215. - PubMed
-
- Undheim EAB, King GF. On the venom system of centipedes (Chilopoda), a neglected group of venomous animals. Toxicon. 2011;57:512–524. - PubMed
-
- McCue MD. Enzyme activities and biological functions of snake venoms. Appl Herpetol. 2005;2:109–123.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

