Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer's disease
- PMID: 29358399
- PMCID: PMC5819390
- DOI: 10.1073/pnas.1710329115
Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer's disease
Abstract
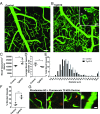
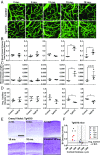
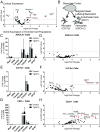
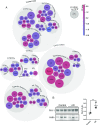
Mixed pathology, with both Alzheimer's disease and vascular abnormalities, is the most common cause of clinical dementia in the elderly. While usually thought to be concurrent diseases, the fact that changes in cerebral blood flow are a prominent early and persistent alteration in Alzheimer's disease raises the possibility that vascular alterations and Alzheimer pathology are more directly linked. Here, we report that aged tau-overexpressing mice develop changes to blood vessels including abnormal, spiraling morphologies; reduced blood vessel diameters; and increased overall blood vessel density in cortex. Blood flow in these vessels was altered, with periods of obstructed flow rarely observed in normal capillaries. These changes were accompanied by cortical atrophy as well as increased expression of angiogenesis-related genes such as Vegfa, Serpine1, and Plau in CD31-positive endothelial cells. Interestingly, mice overexpressing nonmutant forms of tau in the absence of frank neurodegeneration also demonstrated similar changes. Furthermore, many of the genes we observe in mice are also altered in human RNA datasets from Alzheimer patients, particularly in brain regions classically associated with tau pathology such as the temporal lobe and limbic system regions. Together these data indicate that tau pathological changes in neurons can impact brain endothelial cell biology, altering the integrity of the brain's microvasculature.
Keywords: Alzheimer’s disease; angiogenesis; blood vessels; brain microvessels; tau.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

