Molecular clutch drives cell response to surface viscosity
- PMID: 29358406
- PMCID: PMC5819391
- DOI: 10.1073/pnas.1710653115
Molecular clutch drives cell response to surface viscosity
Abstract
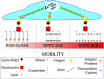
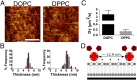
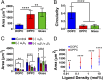
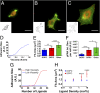
Cell response to matrix rigidity has been explained by the mechanical properties of the actin-talin-integrin-fibronectin clutch. Here the molecular clutch model is extended to account for cell interactions with purely viscous surfaces (i.e., without an elastic component). Supported lipid bilayers present an idealized and controllable system through which to study this concept. Using lipids of different diffusion coefficients, the mobility (i.e., surface viscosity) of the presented ligands (in this case RGD) was altered by an order of magnitude. Cell size and cytoskeletal organization were proportional to viscosity. Furthermore, there was a higher number of focal adhesions and a higher phosphorylation of FAK on less-mobile (more-viscous) surfaces. Actin retrograde flow, an indicator of the force exerted on surfaces, was also seen to be faster on more mobile surfaces. This has consequential effects on downstream molecules; the mechanosensitive YAP protein localized to the nucleus more on less-mobile (more-viscous) surfaces and differentiation of myoblast cells was enhanced on higher viscosity. This behavior was explained within the framework of the molecular clutch model, with lower viscosity leading to a low force loading rate, preventing the exposure of mechanosensitive proteins, and with a higher viscosity causing a higher force loading rate exposing these sites, activating downstream pathways. Consequently, the understanding of how viscosity (regardless of matrix stiffness) influences cell response adds a further tool to engineer materials that control cell behavior.
Keywords: cell differentiation; matrix rigidity; mechanotransduction; molecular clutch; surface viscosity.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. - PubMed
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
-
- Dalby MJ, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. - PubMed
-
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

