OxyR-Dependent Transcription Response of Sinorhizobium meliloti to Oxidative Stress
- PMID: 29358497
- PMCID: PMC5847651
- DOI: 10.1128/JB.00622-17
OxyR-Dependent Transcription Response of Sinorhizobium meliloti to Oxidative Stress
Abstract
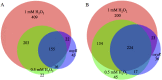
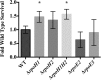
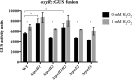
Reactive oxygen species such as peroxides play an important role in plant development, cell wall maturation, and defense responses. During nodulation with the host plant Medicago sativa, Sinorhizobium meliloti cells are exposed to H2O2 in infection threads and developing nodules (R. Santos, D. Hérouart, S. Sigaud, D. Touati, and A. Puppo, Mol Plant Microbe Interact 14:86-89, 2001, https://doi.org/10.1094/MPMI.2001.14.1.86). S. meliloti cells likely also experience oxidative stress, from both internal and external sources, during life in the soil. Here, we present microarray transcription data for S. meliloti wild-type cells compared to a mutant deficient in the key oxidative regulatory protein OxyR, each in response to H2O2 treatment. Several alternative sigma factor genes are upregulated in the response to H2O2; the stress sigma gene rpoE2 shows OxyR-dependent induction by H2O2, while rpoH1 expression is induced by H2O2 irrespective of the oxyR genotype. The activity of the RpoE2 sigma factor in turn causes increased expression of two more sigma factor genes, rpoE5 and rpoH2 Strains with deletions of rpoH1 showed improved survival in H2O2 as well as increased levels of oxyR and total catalase expression. These results imply that ΔrpoH1 strains are primed to deal with oxidative stress. This work presents a global view of S. meliloti gene expression changes, and of regulation of those changes, in response to H2O2IMPORTANCE Like all aerobic organisms, the symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti experiences oxidative stress throughout its complex life cycle. This report describes the global transcriptional changes that S. meliloti makes in response to H2O2 and the roles of the OxyR transcriptional regulator and the RpoH1 sigma factor in regulating those changes. By understanding the complex regulatory response of S. meliloti to oxidative stress, we may further understand the role that reactive oxygen species play as both stressors and potential signals during symbiosis.
Keywords: OxyR; RpoH; Sinorhizobium meliloti; catalase; oxidative stress; sigma factors; transcriptome.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

