An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction
- PMID: 29358595
- PMCID: PMC5778078
- DOI: 10.1038/s41598-018-19906-w
An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction
Abstract
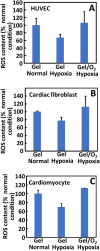
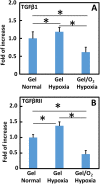
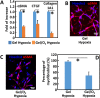
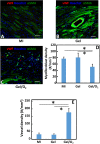
Oxygen deficiency after myocardial infarction (MI) leads to massive cardiac cell death. Protection of cardiac cells and promotion of cardiac repair are key therapeutic goals. These goals may be achieved by re-introducing oxygen into the infarcted area. Yet current systemic oxygen delivery approaches cannot efficiently diffuse oxygen into the infarcted area that has extremely low blood flow. In this work, we developed a new oxygen delivery system that can be delivered specifically to the infarcted tissue, and continuously release oxygen to protect the cardiac cells. The system was based on a thermosensitive, injectable and fast gelation hydrogel, and oxygen releasing microspheres. The fast gelation hydrogel was used to increase microsphere retention in the heart tissue. The system was able to continuously release oxygen for 4 weeks. The released oxygen significantly increased survival of cardiac cells under the hypoxic condition (1% O2) mimicking that of the infarcted hearts. It also reduced myofibroblast formation under hypoxic condition (1% O2). After implanting into infarcted hearts for 4 weeks, the released oxygen significantly augmented cell survival, decreased macrophage density, reduced collagen deposition and myofibroblast density, and stimulated tissue angiogenesis, leading to a significant increase in cardiac function.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









Similar articles
-
Spatiotemporal delivery of basic fibroblast growth factor to directly and simultaneously attenuate cardiac fibrosis and promote cardiac tissue vascularization following myocardial infarction.J Control Release. 2019 Oct;311-312:233-244. doi: 10.1016/j.jconrel.2019.09.005. Epub 2019 Sep 12. J Control Release. 2019. PMID: 31521744 Free PMC article.
-
An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition.Biomaterials. 2012 Sep;33(25):5914-23. doi: 10.1016/j.biomaterials.2012.05.012. Epub 2012 May 30. Biomaterials. 2012. PMID: 22656447
-
Improving therapeutic effects of exosomes encapsulated gelatin methacryloyl/hyaluronic acid blended and oxygen releasing injectable hydrogel by cardiomyocytes induction and vascularization in rat myocardial infarction model.Int J Biol Macromol. 2024 Jun;271(Pt 2):132412. doi: 10.1016/j.ijbiomac.2024.132412. Epub 2024 May 15. Int J Biol Macromol. 2024. PMID: 38754674
-
Progresses and perspectives on natural polysaccharide based hydrogels for repair of infarcted myocardium.Int J Biol Macromol. 2024 Jun;269(Pt 2):132213. doi: 10.1016/j.ijbiomac.2024.132213. Epub 2024 May 8. Int J Biol Macromol. 2024. PMID: 38729464 Review.
-
Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction.Expert Opin Drug Deliv. 2013 Jan;10(1):59-72. doi: 10.1517/17425247.2013.739156. Epub 2012 Nov 9. Expert Opin Drug Deliv. 2013. PMID: 23140533 Review.
Cited by
-
Nanotechnology in cardiac stem cell therapy: cell modulation, imaging and gene delivery.RSC Adv. 2021 Oct 26;11(55):34572-34588. doi: 10.1039/d1ra06404e. eCollection 2021 Oct 25. RSC Adv. 2021. PMID: 35494731 Free PMC article. Review.
-
Optimizing an Injectable Composite Oxygen-Generating System for Relieving Tissue Hypoxia.Front Bioeng Biotechnol. 2020 May 26;8:511. doi: 10.3389/fbioe.2020.00511. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32528945 Free PMC article.
-
Thermosensitive, fast gelling, photoluminescent, highly flexible, and degradable hydrogels for stem cell delivery.Acta Biomater. 2019 Jan 1;83:96-108. doi: 10.1016/j.actbio.2018.10.038. Epub 2018 Oct 26. Acta Biomater. 2019. PMID: 30541703 Free PMC article.
-
Resolution of Inflammation after Skeletal Muscle Ischemia-Reperfusion Injury: A Focus on the Lipid Mediators Lipoxins, Resolvins, Protectins and Maresins.Antioxidants (Basel). 2022 Jun 20;11(6):1213. doi: 10.3390/antiox11061213. Antioxidants (Basel). 2022. PMID: 35740110 Free PMC article. Review.
-
Injectable Hydrogel-Based Nanocomposites for Cardiovascular Diseases.Front Bioeng Biotechnol. 2020 Mar 31;8:251. doi: 10.3389/fbioe.2020.00251. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32296694 Free PMC article. Review.
References
-
- Li Z, Guan J. Hydrogels for Cardiac Tissue Engineering. Polymers. 2011;3:740–761. doi: 10.3390/polym3020740. - DOI
-
- Tsuruda T, Costello-Boerrigter LC, Burnett JC., Jr. Matrix metalloproteinases: pathways of induction by bioactive molecules. Heart failure reviews. 2004;9:53–61. doi: 10.1023/B:HREV.0000011394.34355.bb. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

