Long-acting injectable atovaquone nanomedicines for malaria prophylaxis
- PMID: 29358624
- PMCID: PMC5778127
- DOI: 10.1038/s41467-017-02603-z
Long-acting injectable atovaquone nanomedicines for malaria prophylaxis
Abstract
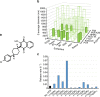
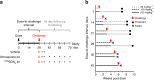
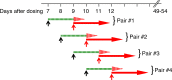
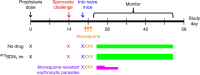
Chemoprophylaxis is currently the best available prevention from malaria, but its efficacy is compromised by non-adherence to medication. Here we develop a long-acting injectable formulation of atovaquone solid drug nanoparticles that confers long-lived prophylaxis against Plasmodium berghei ANKA malaria in C57BL/6 mice. Protection is obtained at plasma concentrations above 200 ng ml-1 and is causal, attributable to drug activity against liver stage parasites. Parasites that appear after subtherapeutic doses remain atovaquone-sensitive. Pharmacokinetic-pharmacodynamic analysis indicates protection can translate to humans at clinically achievable and safe drug concentrations, potentially offering protection for at least 1 month after a single administration. These findings support the use of long-acting injectable formulations as a new approach for malaria prophylaxis in travellers and for malaria control in the field.
Conflict of interest statement
The authors declare no competing financial interests. S.P.R., A.O., A.C.S., L.T.,T.A.S., R.P.B., G.M. and A.K.T. are inventors on a patent filing describing the use of atovaquone SDNs.
Figures

Similar articles
-
Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes.Science. 2016 Apr 15;352(6283):349-53. doi: 10.1126/science.aad9279. Science. 2016. PMID: 27081071 Free PMC article.
-
Within-Host Selection of Drug Resistance in a Mouse Model Reveals Dose-Dependent Selection of Atovaquone Resistance Mutations.Antimicrob Agents Chemother. 2017 Apr 24;61(5):e01867-16. doi: 10.1128/AAC.01867-16. Print 2017 May. Antimicrob Agents Chemother. 2017. PMID: 28193656 Free PMC article.
-
Effectiveness of twice a week prophylaxis with atovaquone-proguanil (Malarone®) in long-term travellers to West Africa.J Travel Med. 2016 Sep 13;23(6):taw064. doi: 10.1093/jtm/taw064. Print 2016 Jun. J Travel Med. 2016. PMID: 27625401
-
Atovaquone and proguanil hydrochloride for prophylaxis of malaria.J Travel Med. 1999 May;6 Suppl 1:S21-7. J Travel Med. 1999. PMID: 23573549 Review.
-
Atovaquone and proguanil hydrochloride: a review of nonclinical studies.J Travel Med. 1999 May;6 Suppl 1:S8-12. J Travel Med. 1999. PMID: 23573546 Review.
Cited by
-
Polymer-prodrug conjugates as candidates for degradable, long-acting implants, releasing the water-soluble nucleoside reverse-transcriptase inhibitor emtricitabine.J Mater Chem B. 2023 Dec 13;11(48):11532-11543. doi: 10.1039/d3tb02268d. J Mater Chem B. 2023. PMID: 37955203 Free PMC article.
-
Linear and branched polymer prodrugs of the water-soluble nucleoside reverse-transcriptase inhibitor emtricitabine as structural materials for long-acting implants.J Mater Chem B. 2022 Jun 15;10(23):4395-4404. doi: 10.1039/d2tb00825d. J Mater Chem B. 2022. PMID: 35604111 Free PMC article.
-
Preferences of Patients and Providers in High-Burden Malaria Settings for Long-Acting Malaria Chemoprevention.Am J Trop Med Hyg. 2023 Aug 21;109(4):752-760. doi: 10.4269/ajtmh.23-0245. Print 2023 Oct 4. Am J Trop Med Hyg. 2023. PMID: 37604474 Free PMC article.
-
Antimalarial drug discovery: progress and approaches.Nat Rev Drug Discov. 2023 Oct;22(10):807-826. doi: 10.1038/s41573-023-00772-9. Epub 2023 Aug 31. Nat Rev Drug Discov. 2023. PMID: 37652975 Free PMC article. Review.
-
What Clinicians Need to Know About the Development of Long-Acting Formulations.Clin Infect Dis. 2022 Nov 21;75(Suppl 4):S487-S489. doi: 10.1093/cid/ciac749. Clin Infect Dis. 2022. PMID: 36410382 Free PMC article.
References
-
- WHO. World Health Organization Fact Sheet: World Malaria Report 2016 (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

