Long-acting injectable atovaquone nanomedicines for malaria prophylaxis
- PMID: 29358624
- PMCID: PMC5778127
- DOI: 10.1038/s41467-017-02603-z
Long-acting injectable atovaquone nanomedicines for malaria prophylaxis
Abstract
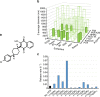
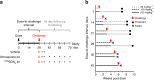
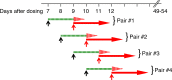
Chemoprophylaxis is currently the best available prevention from malaria, but its efficacy is compromised by non-adherence to medication. Here we develop a long-acting injectable formulation of atovaquone solid drug nanoparticles that confers long-lived prophylaxis against Plasmodium berghei ANKA malaria in C57BL/6 mice. Protection is obtained at plasma concentrations above 200 ng ml-1 and is causal, attributable to drug activity against liver stage parasites. Parasites that appear after subtherapeutic doses remain atovaquone-sensitive. Pharmacokinetic-pharmacodynamic analysis indicates protection can translate to humans at clinically achievable and safe drug concentrations, potentially offering protection for at least 1 month after a single administration. These findings support the use of long-acting injectable formulations as a new approach for malaria prophylaxis in travellers and for malaria control in the field.
Conflict of interest statement
The authors declare no competing financial interests. S.P.R., A.O., A.C.S., L.T.,T.A.S., R.P.B., G.M. and A.K.T. are inventors on a patent filing describing the use of atovaquone SDNs.
Figures

References
-
- WHO. World Health Organization Fact Sheet: World Malaria Report 2016 (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

