Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations
- PMID: 29358889
- PMCID: PMC5757117
- DOI: 10.3748/wjg.v24.i1.124
Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations
Abstract
Aim: To summarize and compare worldwide colorectal cancer (CRC) screening recommendations in order to identify similarities and disparities.
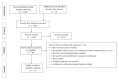
Methods: A systematic literature search was performed using MEDLINE, EMBASE, Scopus, CENTRAL and ISI Web of knowledge identifying all average-risk CRC screening guideline publications within the last ten years and/or position statements published in the last 2 years. In addition, a hand-search of the webpages of National Gastroenterology Society websites, the National Guideline Clearinghouse, the BMJ Clinical Evidence website, Google and Google Scholar was performed.
Results: Fifteen guidelines were identified. Six guidelines were published in North America, four in Europe, four in Asia and one from the World Gastroenterology Organization. The majority of guidelines recommend screening average-risk individuals between ages 50 and 75 using colonoscopy (every 10 years), or flexible sigmoidoscopy (FS, every 5 years) or fecal occult blood test (FOBT, mainly the Fecal Immunochemical Test, annually or biennially). Disparities throughout the different guidelines are found relating to the use of colonoscopy, rank order between test, screening intervals and optimal age ranges for screening.
Conclusion: Average risk individuals between 50 and 75 years should undergo CRC screening. Recommendations for optimal surveillance intervals, preferred tests/test cascade as well as the optimal timing when to start and stop screening differ regionally and should be considered for clinical decision making. Furthermore, local resource availability and patient preferences are important to increase CRC screening uptake, as any screening is better than none.
Keywords: Colonoscopy; Colorectal cancer; Fecal immunochemical test; Fecal occult blood test; Flexible sigmoidoscopy; Guidelines; Screening; Systematic review.
Conflict of interest statement
Conflict-of-interest statement: Florence Bénard has no potential conflict of interest to disclose. Alan Barkun is the lead clinician for the Quebec colorectal cancer screening program and has received consulting honoraria from Olympus. Myriam Martel has no potential conflict of interest to disclose. Daniel von Renteln is supported through a Fonds de recherche du Québec- Santé (FRQS) career development award, has received consulting honoraria from Boston Scientific and has received research support from ERBE, Vantage and Pentax.
References
-
- IARC. Lyon: IARC; 2012. Cancer Fact Sheets: Colorectal Cancer. [cited April 7 2017]. Global Cancer Observatory [Internet] Available from: http://gco.iarc.fr/today/data/pdf/fact-sheets/cancers/cancer-fact-sheets....
-
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674–1680. - PubMed
-
- Kita MW. Reduction in colorectal cancer mortality related to annual fecal occult blood screening--13 year follow-up of 46,000 subjects. J Insur Med. 1993;25:138–139. - PubMed
-
- Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004;39:846–851. - PubMed
-
- Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95:1029–1036. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases