Decoding of Ankle Flexion and Extension from Cortical Current Sources Estimated from Non-invasive Brain Activity Recording Methods
- PMID: 29358903
- PMCID: PMC5766671
- DOI: 10.3389/fnins.2017.00733
Decoding of Ankle Flexion and Extension from Cortical Current Sources Estimated from Non-invasive Brain Activity Recording Methods
Abstract
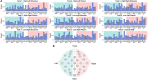
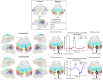
The classification of ankle movements from non-invasive brain recordings can be applied to a brain-computer interface (BCI) to control exoskeletons, prosthesis, and functional electrical stimulators for the benefit of patients with walking impairments. In this research, ankle flexion and extension tasks at two force levels in both legs, were classified from cortical current sources estimated by a hierarchical variational Bayesian method, using electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) recordings. The hierarchical prior for the current source estimation from EEG was obtained from activated brain areas and their intensities from an fMRI group (second-level) analysis. The fMRI group analysis was performed on regions of interest defined over the primary motor cortex, the supplementary motor area, and the somatosensory area, which are well-known to contribute to movement control. A sparse logistic regression method was applied for a nine-class classification (eight active tasks and a resting control task) obtaining a mean accuracy of 65.64% for time series of current sources, estimated from the EEG and the fMRI signals using a variational Bayesian method, and a mean accuracy of 22.19% for the classification of the pre-processed of EEG sensor signals, with a chance level of 11.11%. The higher classification accuracy of current sources, when compared to EEG classification accuracy, was attributed to the high number of sources and the different signal patterns obtained in the same vertex for different motor tasks. Since the inverse filter estimation for current sources can be done offline with the present method, the present method is applicable to real-time BCIs. Finally, due to the highly enhanced spatial distribution of current sources over the brain cortex, this method has the potential to identify activation patterns to design BCIs for the control of an affected limb in patients with stroke, or BCIs from motor imagery in patients with spinal cord injury.
Keywords: brain computer interface; electroencephalography; functional magnetic resonance imaging; walking.
Figures



References
-
- Amaral D. G. (2013). The functional organization of perception and movement, in Principles of Neural Science, eds Kandel E. R., Schwartz J. H., Jessell T. M. (New York, NY: McGraw-Hill Companies; ), 356–369.
-
- Attias H. (1999). Inferring parameters and structure of latent variable models by variational Bayes. Proc. Fifteenth Conf. Uncertain. Artif. Intell. 1–5. 10.1007/s13398-014-0173-7.2 - DOI
-
- Baillet S., Mosher J. C., Leahy R. M. (2001). Electromagnetic brain mapping. IEEE Signal Process. Mag. 18, 14–30. 10.1109/79.962275 - DOI
-
- Barsotti M., Leonardis D., Loconsole C., Solazzi M., Sotgiu E., Procopio C., et al. (2015). A full upper limb robotic exoskeleton for reaching and grasping rehabilitation triggered by MI-BCI, in IEEE International Conference Rehabilitative Robotics 2015–September (Singapore: ), 49–54.
LinkOut - more resources
Full Text Sources
Other Literature Sources

