Compact, multi-exposure speckle contrast optical spectroscopy (SCOS) device for measuring deep tissue blood flow
- PMID: 29359106
- PMCID: PMC5772585
- DOI: 10.1364/BOE.9.000322
Compact, multi-exposure speckle contrast optical spectroscopy (SCOS) device for measuring deep tissue blood flow
Abstract
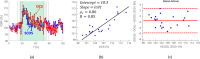
Speckle contrast optical spectroscopy (SCOS) measures absolute blood flow in deep tissue, by taking advantage of multi-distance (previously reported in the literature) or multi-exposure (reported here) approach. This method promises to use inexpensive detectors to obtain good signal-to-noise ratio, but it has not yet been implemented in a suitable manner for a mass production. Here we present a new, compact, low power consumption, 32 by 2 single photon avalanche diode (SPAD) array that has no readout noise, low dead time and has high sensitivity in low light conditions, such as in vivo measurements. To demonstrate the capability to measure blood flow in deep tissue, healthy volunteers were measured, showing no significant differences from the diffuse correlation spectroscopy. In the future, this array can be miniaturized to a low-cost, robust, battery operated wireless device paving the way for measuring blood flow in a wide-range of applications from sport injury recovery and training to, on-field concussion detection to wearables.
Keywords: (170.0170) Medical optics and biotechnology; (170.1470) Blood or tissue constituent monitoring; (170.3890) Medical optics instrumentation; (300.6480) Spectroscopy, speckle.
Conflict of interest statement
ICFO has equity ownership in the spin-off company HemoPhotonics S.L. that commercializes DCS technology. Potential financial conflicts of interest and objectivity of research have been monitored by ICFO’s Knowledge & Technology Transfer Department. No financial conflicts of interest were identified or declared by the authors.
Figures
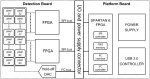
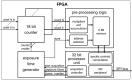


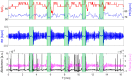
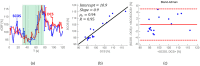
References
-
- Zauner A., Muizelaar J. P., “Brain metabolism and cerebral blood flow,” Head Inj. 1997 89–99 (1997).
-
- Devor A., Sakadžić S., Srinivasan V. J., Yaseen M. A., Nizar K., Saisan P. A., Tian P., Dale A. M., Vinogradov S. A., Franceschini M. A., et al. , “Frontiers in optical imaging of cerebral blood flow and metabolism,” J. Cereb. Blood Flow Metab. 32, 1259–1276 (2012).10.1038/jcbfm.2011.195 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
