Immune Response and Protective Efficacy of a Heterologous DNA-Protein Immunization with Leishmania Superoxide Dismutase B1
- PMID: 29359145
- PMCID: PMC5735611
- DOI: 10.1155/2017/2145386
Immune Response and Protective Efficacy of a Heterologous DNA-Protein Immunization with Leishmania Superoxide Dismutase B1
Abstract
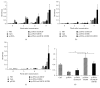
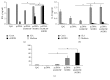
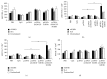
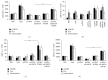
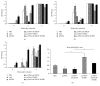
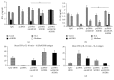
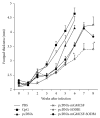
Growing evidence shows that antioxidant proteins of Leishmania could be used as vaccine candidates. In this study, we report the efficacy of Leishmania donovani iron superoxide dismutase B1 (LdFeSODB1) as a vaccine antigen in BALB/c mice in a DNA-protein prime-boost immunization regimen in the presence or absence of murine granulocyte macrophage colony stimulating factor (mGMCSF) DNA adjuvant. The expression study confirmed that LdFeSODB1 is expressed in mammalian cells and mGMCSF fusion mediates the secretion of the recombinant protein. Heterologous immunization with LdFeSODB1 induced a strong antibody- and cell-mediated immune response in mice. Immunization triggered a mixed Th1/Th2 response as evidenced by the ratio of IgG2a to IgG1. Antigen-stimulated spleen cells from the immunized mice produced high level IFN-γ. Multiparametric flow cytometry data showed that immunization with LdFeSODB1 induced significantly higher expression of TNF-α or IL-2 by antigen-stimulated T cells. Eight weeks after L. major infection, immunization with the antigen shifted the immune response to a more Th1 type than the controls as demonstrated by IgG2a/IgG1 ratio. Moreover, IFN-γ production by antigen-stimulated spleen cells from immunized mice remained high. The footpad swelling experiment showed that immunization with LdFeSODB1 resulted in partial protection of mice from a high dose L. major infection.
Figures

Similar articles
-
DNA-protein immunization using Leishmania peroxidoxin-1 induces a strong CD4+ T cell response and partially protects mice from cutaneous leishmaniasis: role of fusion murine granulocyte-macrophage colony-stimulating factor DNA adjuvant.PLoS Negl Trop Dis. 2014 Dec 11;8(12):e3391. doi: 10.1371/journal.pntd.0003391. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25500571 Free PMC article.
-
Leishmania donovani recombinant iron superoxide dismutase B1 protein in the presence of TLR-based adjuvants induces partial protection of BALB/c mice against Leishmania major infection.Exp Parasitol. 2012 Jul;131(3):317-24. doi: 10.1016/j.exppara.2012.05.002. Epub 2012 May 10. Exp Parasitol. 2012. PMID: 22580023
-
Heterologous priming-boosting with DNA and vaccinia virus expressing kinetoplastid membrane protein-11 induces potent cellular immune response and confers protection against infection with antimony resistant and sensitive strains of Leishmania (Leishmania) donovani.Vaccine. 2013 Apr 8;31(15):1905-15. doi: 10.1016/j.vaccine.2013.02.025. Epub 2013 Feb 26. Vaccine. 2013. PMID: 23499564
-
Potency, efficacy and durability of DNA/DNA, DNA/protein and protein/protein based vaccination using gp63 against Leishmania donovani in BALB/c mice.PLoS One. 2011 Feb 2;6(2):e14644. doi: 10.1371/journal.pone.0014644. PLoS One. 2011. PMID: 21311597 Free PMC article.
-
Leishmaniasis: current status of vaccine development.Curr Mol Med. 2004 Sep;4(6):667-79. doi: 10.2174/1566524043360203. Curr Mol Med. 2004. PMID: 15357215 Review.
Cited by
-
Immunization with recombinant LiHyp1 protein plus adjuvant is protective against tegumentary leishmaniasis.Parasitol Res. 2023 Dec;122(12):2917-2931. doi: 10.1007/s00436-023-07981-6. Epub 2023 Sep 28. Parasitol Res. 2023. PMID: 37768367
References
-
- WHO. WHO, Third WHO Report on Neglected Tropical Diseases, 2015.
-
- Khamesipour A., Rafati S., Davoudi N., Maboudi F., Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian Journal of Medical Research. 2006;123(3):423–438. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

