Cholinergic interneurons in the rat striatum modulate substitution of habits
- PMID: 29359362
- PMCID: PMC6001626
- DOI: 10.1111/ejn.13820
Cholinergic interneurons in the rat striatum modulate substitution of habits
Abstract
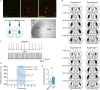
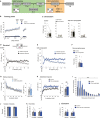
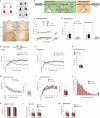
Behavioural flexibility is crucial for adaptive behaviour, and recent evidence suggests that cholinergic interneurons of the striatum play a distinct role. Previous studies of cholinergic function have focused on strategy switching by the dorsomedial or ventral striatum. We here investigated whether cholinergic interneurons in the dorsolateral striatum play a similar role at the level of switching of habitual responses. Because the dorsolateral striatum is particularly involved in habitual responding, we developed a habit substitution task that involved switching habitual lever-press responses to one side to another. We first measured the effect of cholinergic activation in the dorsolateral striatum on this task. Chemogenetic activation of cholinergic interneurons caused an increase in the response rate for the substituted response that was significantly greater than the increase normally seen in control animals. The increase was due to burst-like responses with shorter inter-press intervals. However, there was no effect on inhibiting the old habit, or on habitual responding that did not require a switch. There was also no effect on lever-press performance and its reversal before lever-press responses became habitual. Conversely, neurochemically specific ablation of cholinergic interneurons did not significantly change habitual responding or response substitution. Thus, activation -but not ablation -of cholinergic interneurons in the dorsolateral striatum modulates expression of a new habit when an old habit is replaced by a new one. Together with previous work, this suggests that striatal cholinergic interneurons facilitate behavioural flexibility in both dorsolateral striatum in addition to dorsomedial and ventral striatum.
Keywords: acetylcholine; basal ganglia; behavioural flexibility; chemogenetics.
© 2018 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures



References
-
- Aosaki, T. , Kimura, M. & Graybiel, A.M. (1995) Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J. Neurophysiol., 73, 1234–1252. - PubMed
-
- Apicella, P. , Deffains, M. , Ravel, S. & Legallet, E. (2009) Tonically active neurons in the striatum differentiate between delivery and omission of expected reward in a probabilistic task context. Eur. J. Neurosci., 30, 515–526. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

