Modern Electrochemical Aspects for the Synthesis of Value-Added Organic Products
- PMID: 29359378
- PMCID: PMC6001547
- DOI: 10.1002/anie.201712732
Modern Electrochemical Aspects for the Synthesis of Value-Added Organic Products
Abstract
The use of electricity instead of stoichiometric amounts of oxidizers or reducing agents in synthesis is very appealing for economic and ecological reasons, and represents a major driving force for research efforts in this area. To use electron transfer at the electrode for a successful transformation in organic synthesis, the intermediate radical (cation/anion) has to be stabilized. Its combination with other approaches in organic chemistry or concepts of contemporary synthesis allows the establishment of powerful synthetic methods. The aim in the 21st Century will be to use as little fossil carbon as possible and, for this reason, the use of renewable sources is becoming increasingly important. The direct conversion of renewables, which have previously mainly been incinerated, is of increasing interest. This Review surveys many of the recent seminal important developments which will determine the future of this dynamic emerging field.
Keywords: electrolysis; flow electrochemistry; organocatalysis; renewable resources; synthetic methods.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

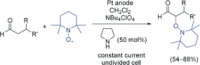



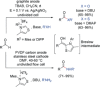

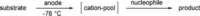


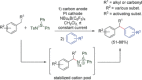
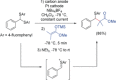


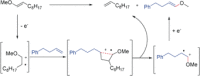

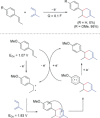



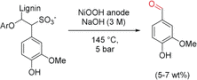



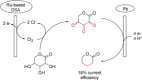

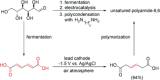
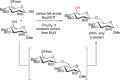




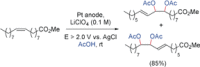
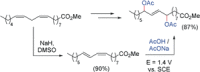
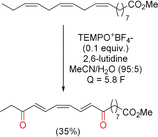
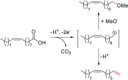




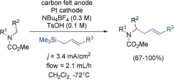



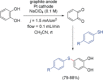



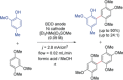

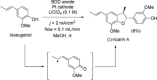
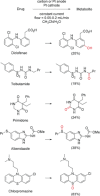

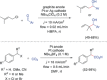
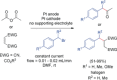


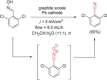

References
-
- None
-
- Waldvogel S. R., Möhle S., Angew. Chem. Int. Ed. 2015, 54, 6398–6399; - PubMed
- Angew. Chem. 2015, 127, 6496–6497;
-
- Waldvogel S. R., Selt M., Angew. Chem. Int. Ed. 2016, 55, 12578–12580; - PubMed
- Angew. Chem. 2016, 128, 12766–12768;
-
- Waldvogel S. R., Janza B., Angew. Chem. Int. Ed. 2014, 53, 7122–7123; - PubMed
- Angew. Chem. 2014, 126, 7248–7249;
-
- Schäfer H. J., Angew. Chem. Int. Ed. 2017, 56, 15502–15503; - PubMed
- Angew. Chem. 2017, 129, 15706–15708.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

