Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis
- PMID: 29359519
- PMCID: PMC5814341
- DOI: 10.1111/apt.14510
Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis
Abstract
Background: Vedolizumab is an effective therapy for ulcerative colitis (UC), but costly and slow to work. New clinical responses occur after 30 weeks of therapy.
Aims: To enable physicians, patients, and insurers to predict whether a patient with UC will respond to vedolizumab at an early time point after starting therapy.
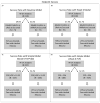
Methods: The clinical study data request website provided the phase 3 clinical trial data for vedolizumab. Random forest models were trained on 70% and tested on 30% of the data to predict corticosteroid-free endoscopic remission at week 52. Models were constructed using baseline data, or data through week 6 of vedolizumab therapy from 491 subjects.
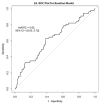
Results: The AuROC for prediction of corticosteroid-free endoscopic remission at week 52 using baseline data was only 0.62 (95% CI: 0.53-0.72), but was 0.73 (95% CI: 0.65-0.82) when using data through week 6. A total of 47% of subjects were predicted to be remitters, and 59% of these subjects achieved corticosteroid-free endoscopic remission, in contrast to 21% of the predicted non-remitters. A week 6 prediction using FCP ≤234 μg/g was nearly as accurate.
Conclusions: A machine learning algorithm using laboratory data through week 6 of vedolizumab therapy was able to accurately identify which UC patients would achieve corticosteroid-free endoscopic remission on vedolizumab at week 52. Application of this algorithm could have significant implications for clinical decisions on whom to continue on this costly medication when the benefits of the vedolizumab are not clinically apparent in the first 6 weeks of therapy.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
Peter D.R. Higgins has served as a consultant for Takeda Pharmaceuticals International, Inc. All other authors have no personal interests to declare.
Akbar K. Waljee is supported by a career development grant award (CDA 11-217) from the United States Department of Veterans Affairs Health Services Research and Development Service. Peter D.R. Higgin’s and Akbar K. Waljee’s research is supported by NIH R01 GM097117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the University of Michigan, the Veterans Affairs, or the National Institutes of Health.
Figures



References
-
- Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
