Biocatalytic site- and enantioselective oxidative dearomatization of phenols
- PMID: 29359749
- PMCID: PMC6503525
- DOI: 10.1038/nchem.2879
Biocatalytic site- and enantioselective oxidative dearomatization of phenols
Abstract
The biocatalytic transformations used by chemists are often restricted to simple functional-group interconversions. In contrast, nature has developed complexity-generating biocatalytic reactions within natural product pathways. These sophisticated catalysts are rarely employed by chemists, because the substrate scope, selectivity and robustness of these catalysts are unknown. Our strategy to bridge the gap between the biosynthesis and synthetic chemistry communities leverages the diversity of catalysts available within natural product pathways. Here we show that, starting from a suite of biosynthetic enzymes, catalysts with complementary substrate scope as well as selectivity can be identified. This strategy has been applied to the oxidative dearomatization of phenols, a chemical transformation that rapidly builds molecular complexity from simple starting materials and cannot be accomplished with high selectivity using existing catalytic methods. Using enzymes from biosynthetic pathways, we have successfully developed a method to produce ortho-quinol products with controlled site- and stereoselectivity. Furthermore, we have capitalized on the scalability and robustness of this method in gram-scale reactions as well as multi-enzyme and chemoenzymatic cascades.
Figures

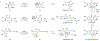

References
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/347750943
- PubChem-Substance/347750980
- PubChem-Substance/347750944
- PubChem-Substance/347750945
- PubChem-Substance/347750946
- PubChem-Substance/347750947
- PubChem-Substance/347750948
- PubChem-Substance/347750949
- PubChem-Substance/347750950
- PubChem-Substance/347750951
- PubChem-Substance/347750981
- PubChem-Substance/347750952
- PubChem-Substance/347750982
- PubChem-Substance/347750953
- PubChem-Substance/347750983
- PubChem-Substance/347750954
- PubChem-Substance/347750984
- PubChem-Substance/347750955
- PubChem-Substance/347750985
- PubChem-Substance/347750956
- PubChem-Substance/347750986
- PubChem-Substance/347750957
- PubChem-Substance/347750987
- PubChem-Substance/347750958
- PubChem-Substance/347750988
- PubChem-Substance/347750959
- PubChem-Substance/347750989
- PubChem-Substance/347750960
- PubChem-Substance/347750990
- PubChem-Substance/347750961
- PubChem-Substance/347750991
- PubChem-Substance/347750962
- PubChem-Substance/347750992
- PubChem-Substance/347750963
- PubChem-Substance/347750993
- PubChem-Substance/347750964
- PubChem-Substance/347750994
- PubChem-Substance/347750965
- PubChem-Substance/347750995
- PubChem-Substance/347750966
- PubChem-Substance/347750996
- PubChem-Substance/347750967
- PubChem-Substance/347750968
- PubChem-Substance/347750969
- PubChem-Substance/347750970
- PubChem-Substance/347750971
- PubChem-Substance/347750972
- PubChem-Substance/347750973
- PubChem-Substance/347750974
- PubChem-Substance/347750975
- PubChem-Substance/347750976
- PubChem-Substance/347750977
- PubChem-Substance/347750978
- PubChem-Substance/347751010
- PubChem-Substance/347751011
- PubChem-Substance/347751012
- PubChem-Substance/347750979
- PubChem-Substance/347751013
- PubChem-Substance/347751014
- PubChem-Substance/347751015
- PubChem-Substance/347751016
- PubChem-Substance/347751017
- PubChem-Substance/347751019
- PubChem-Substance/347751020
- PubChem-Substance/347751021
- PubChem-Substance/347751018
- PubChem-Substance/347751022
- PubChem-Substance/347751023
- PubChem-Substance/347751024
- PubChem-Substance/347751025
- PubChem-Substance/347751026
- PubChem-Substance/347751027
- PubChem-Substance/347751028
- PubChem-Substance/347751029
- PubChem-Substance/347751030
- PubChem-Substance/347751031
- PubChem-Substance/347751032
- PubChem-Substance/347751033
- PubChem-Substance/347751034
- PubChem-Substance/347750997
- PubChem-Substance/347751002
- PubChem-Substance/347751003
- PubChem-Substance/347751004
- PubChem-Substance/347751005
- PubChem-Substance/347751006
- PubChem-Substance/347751007
- PubChem-Substance/347751008
- PubChem-Substance/347751009
- PubChem-Substance/347750998
- PubChem-Substance/347750999
- PubChem-Substance/347751000
- PubChem-Substance/347751001
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

