Size Control and Fluorescence Labeling of Polydopamine Melanin-Mimetic Nanoparticles for Intracellular Imaging
- PMID: 29360110
- PMCID: PMC5774220
- DOI: 10.3390/biomimetics2030017
Size Control and Fluorescence Labeling of Polydopamine Melanin-Mimetic Nanoparticles for Intracellular Imaging
Abstract
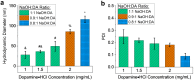
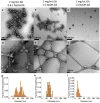
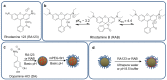
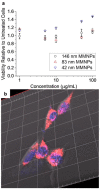
As synthetic analogs of the natural pigment melanin, polydopamine nanoparticles (NPs) are under active investigation as non-toxic anticancer photothermal agents and as free radical scavenging therapeutics. By analogy to the widely adopted polydopamine coatings, polydopamine NPs offer the potential for facile aqueous synthesis and incorporation of (bio)functional groups under mild temperature and pH conditions. However, clear procedures for the convenient and reproducible control of critical NP properties such as particle diameter, surface charge, and loading with functional molecules have yet to be established. In this work, we have synthesized polydopamine-based melanin-mimetic nanoparticles (MMNPs) with finely controlled diameters spanning ≈25 to 120 nm and report on the pH-dependence of zeta potential, methodologies for PEGylation, and the incorporation of fluorescent organic molecules. A comprehensive suite of complementary techniques, including dynamic light scattering (DLS), cryogenic transmission electron microscopy (cryo-TEM), X-ray photoelectron spectroscopy (XPS), zeta-potential, ultraviolet-visible (UV-Vis) absorption and fluorescence spectroscopy, and confocal microscopy, was used to characterize the MMNPs and their properties. Our PEGylated MMNPs are highly stable in both phosphate-buffered saline (PBS) and in cell culture media and exhibit no cytotoxicity up to at least 100 μg mL-1 concentrations. We also show that a post-functionalization methodology for fluorophore loading is especially suitable for producing MMNPs with stable fluorescence and significantly narrower emission profiles than previous reports, suggesting they will be useful for multimodal cell imaging. Our results pave the way towards biomedical imaging and possibly drug delivery applications, as well as fundamental studies of MMNP size and surface chemistry dependent cellular interactions.
Keywords: catechol; dopamine; melanin; nanoparticle.
Conflict of interest statement
Conflicts of Interest The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




References
-
- Gu F.X., Karnik R., Wang A.Z., Alexis F., Levy-Nissenbaum E., Hong S., Langer R.S., Farokhzad O.C. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2:14–21. doi: 10.1016/S1748-0132(07)70083-X. - DOI
-
- De M., Ghosh P.S., Rotello V.M. Applications of nanoparticles in biology. Adv. Mater. 2008;20:4225–4241. doi: 10.1002/adma.200703183. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

