FoxM1 promotes epithelial-mesenchymal transition, invasion, and migration of tongue squamous cell carcinoma cells through a c-Met/AKT-dependent positive feedback loop
- PMID: 29360662
- PMCID: PMC5821477
- DOI: 10.1097/CAD.0000000000000585
FoxM1 promotes epithelial-mesenchymal transition, invasion, and migration of tongue squamous cell carcinoma cells through a c-Met/AKT-dependent positive feedback loop
Abstract
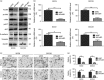
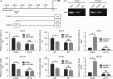
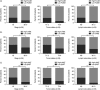
Forkhead box protein M1 (FoxM1) has been associated with cancer progression and metastasis. However, the function of FoxM1 in tongue squamous cell carcinoma (TSCC) remains largely unknown. The purpose of this study was to determine the role of FoxM1 in regulation of epithelial-mesenchymal transition (EMT) and migration of TSCC cells. We found that FoxM1 induced EMT and increased invasion/migration capacity in SCC9 and SCC25 cells. FoxM1 stimulation increased c-Met, pAKT, and vimentin levels but decreased E-cadherin level. Chromatin immunoprecipitation assay established that FoxM1 is bound to the promoter of c-Met to activate its transcription. In turn, c-Met promoted the expression of FoxM1 and pAKT. Blocking AKT signaling attenuated the invasion and migration of SCC9 and SCC25 cells stimulated by FoxM1 or c-Met. These results indicate that a positive feedback loop controls the EMT and migration of TSCC cells induced by FoxM1 and c-Met through AKT. Furthermore, the expression levels of FoxM1, pAKT, and c-Met were found to significantly increase in TSCC tissues compared with normal tissues, and these three biomarkers were concomitantly expressed in TSCC tissues. Clinical association analyses indicated that the expression of FoxM1, c-Met, and pAKT was associated with clinicopathological characteristics of patients with TSCC including tumor stage, tumor size, and lymph node metastasis. Taken together, our findings suggest that FoxM1 promotes the EMT, invasion and migration of TSCC cells, and cross-talks with c-Met/AKT signaling to form a positive feedback loop to promote TSCC development.
Figures




References
-
- Xu H, Liu T, Jing X, Mu J. Dig the root of cancer: targeting cancer stem cells therapy. J Med Discov 2017; 2:JMD17003.
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–132. - PubMed
-
- Torre L, Bray F, Siegel R, Ferlay J, Lortettieulent J, Jemal A. Global cancer statistics, 2002. CA Cancer J Clin 2015; 65:87–108. - PubMed
-
- Angadi P, Patil P, Hallikeri K, Mallapur M, Hallikerimath S, Kale A. Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol 2015; 23:102–110. - PubMed
-
- Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem 2007; 388:1257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

