Clinical Development of PD-1 in Advanced Melanoma
- PMID: 29360722
- PMCID: PMC5819364
- DOI: 10.1097/PPO.0000000000000299
Clinical Development of PD-1 in Advanced Melanoma
Abstract
The development of new treatment options has dramatically improved the landscape for patients with advanced melanoma. Part of these advances emerged through the identification of the importance of factors that regulate the immune system, including proteins that negatively modulate T cell-mediated responses termed "immune checkpoints." Indeed, blockade of the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) immune checkpoint served as a proof of principle that the manipulation of these molecules could induce robust anticancer effects, yet limited to a small percentage of patients. Targeting a distinct checkpoint, the PD-1 yielded improved outcomes and reduced toxicity compared with CTLA-4 blockade and, in separate studies, chemotherapy. More recently, combined CTLA-4/PD-1 blockade was shown to result in higher response rates, while accompanied by increased toxicity. In this article, we review the clinical development of anti-PD-1 monotherapy and combinations that may expand the benefit of immunotherapy for patients with advanced melanoma.
Conflict of interest statement
M.A.P. - honoraria (BMS, Merck); advisory role (BMS, Merck, Novartis, Array BioPharma); research funding (BMS)
R.R.M. - honoraria (BMS, MSD, Roche, Novartis); advisory role (Lilly, Merck Serono, MSD, Roche, Novartis); travel expenses (BMS, MSD, Roche, TEVA, Novartis); research involvement (BMS, Lilly, Roche)
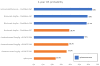
Figures
References
-
- Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–82. - PubMed
-
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. - PubMed
-
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. - PubMed
-
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–1260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

