Temporal requirement of dystroglycan glycosylation during brain development and rescue of severe cortical dysplasia via gene delivery in the fetal stage
- PMID: 29360985
- PMCID: PMC6159531
- DOI: 10.1093/hmg/ddy032
Temporal requirement of dystroglycan glycosylation during brain development and rescue of severe cortical dysplasia via gene delivery in the fetal stage
Abstract
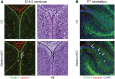
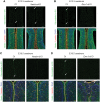
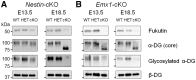
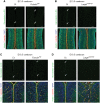
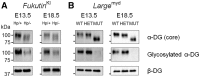
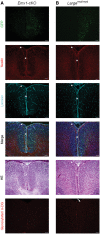
Congenital muscular dystrophies (CMDs) are characterized by progressive weakness and degeneration of skeletal muscle. In several forms of CMD, abnormal glycosylation of α-dystroglycan (α-DG) results in conditions collectively known as dystroglycanopathies, which are associated with central nervous system involvement. We recently demonstrated that fukutin, the gene responsible for Fukuyama congenital muscular dystrophy, encodes the ribitol-phosphate transferase essential for dystroglycan function. Brain pathology in patients with dystroglycanopathy typically includes cobblestone lissencephaly, mental retardation, and refractory epilepsy; however, some patients exhibit average intelligence, with few or almost no structural defects. Currently, there is no effective treatment for dystroglycanopathy, and the mechanisms underlying the generation of this broad clinical spectrum remain unknown. Here, we analysed four distinct mouse models of dystroglycanopathy: two brain-selective fukutin conditional knockout strains (neuronal stem cell-selective Nestin-fukutin-cKO and forebrain-selective Emx1-fukutin-cKO), a FukutinHp strain with the founder retrotransposal insertion in the fukutin gene, and a spontaneous Large-mutant Largemyd strain. These models exhibit variations in the severity of brain pathology, replicating the clinical heterogeneity of dystroglycanopathy. Immunofluorescence analysis of the developing cortex suggested that residual glycosylation of α-DG at embryonic day 13.5 (E13.5), when cortical dysplasia is not yet apparent, may contribute to subsequent phenotypic heterogeneity. Surprisingly, delivery of fukutin or Large into the brains of mice at E12.5 prevented severe brain malformation in Emx1-fukutin-cKO and Largemyd/myd mice, respectively. These findings indicate that spatiotemporal persistence of functionally glycosylated α-DG may be crucial for brain development and modulation of glycosylation during the fetal stage could be a potential therapeutic strategy for dystroglycanopathy.
Figures

References
-
- Muntoni F., Voit T. (2004) The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul. Disord., 14, 635–649. - PubMed
-
- Toda T., Kobayashi K., Takeda S., Sasaki J., Kurahashi H., Kano H., Tachikawa M., Wang F., Nagai Y., Taniguchi K.. et al. (2003) Fukuyama-type congenital muscular dystrophy (FCMD) and alpha-dystroglycanopathy. Congenit. Anom. (Kyoto), 43, 97–104. - PubMed
-
- Michele D.E., Campbell K.P. (2003) Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J. Biol. Chem., 278, 15457–15460. - PubMed
-
- Ibraghimov-Beskrovnaya O., Ervasti J.M., Leveille C.J., Slaughter C.A., Sernett S.W., Campbell K.P. (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature, 355, 696–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

