The female epilepsy protein PCDH19 is a new GABAAR-binding partner that regulates GABAergic transmission as well as migration and morphological maturation of hippocampal neurons
- PMID: 29360992
- PMCID: PMC5886308
- DOI: 10.1093/hmg/ddy019
The female epilepsy protein PCDH19 is a new GABAAR-binding partner that regulates GABAergic transmission as well as migration and morphological maturation of hippocampal neurons
Abstract
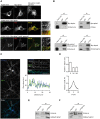
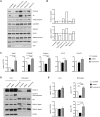
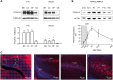
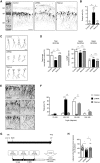
The PCDH19 gene (Xp22.1) encodes the cell-adhesion protein protocadherin-19 (PCDH19) and is responsible for a neurodevelopmental pathology characterized by female-limited epilepsy, cognitive impairment and autistic features, the pathogenic mechanisms of which remain to be elucidated. Here, we identified a new interaction between PCDH19 and GABAA receptor (GABAAR) alpha subunits in the rat brain. PCDH19 shRNA-mediated downregulation reduces GABAAR surface expression and affects the frequency and kinetics of miniature inhibitory postsynaptic currents (mIPSCs) in cultured hippocampal neurons. In vivo, PCDH19 downregulation impairs migration, orientation and dendritic arborization of CA1 hippocampal neurons and increases rat seizure susceptibility. In sum, these data indicate a role for PCDH19 in GABAergic transmission as well as migration and morphological maturation of neurons.
Figures


References
-
- Camacho A., Simón R., Sanz R., Viñuela A., Martínez-Salio A., Mateos F. (2012) Cognitive and behavioral profile in females with epilepsy with PDCH19 mutation: two novel mutations and review of the literature. Epilepsy Behav., 24, 134–137. - PubMed
-
- Duszyc K., Terczynska I., Hoffman-Zacharska D. (2015) Epilepsy and mental retardation restricted to females: X-linked epileptic infantile encephalopathy of unusual inheritance. J. Appl. Genet., 56, 49–56. - PubMed
-
- Wolverton T., Lalande M. (2001) Identification and characterization of three members of a novel subclass of protocadherins. Genomics, 76, 66–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

