Single-particle trajectories reveal two-state diffusion-kinetics of hOGG1 proteins on DNA
- PMID: 29361033
- PMCID: PMC5861423
- DOI: 10.1093/nar/gky004
Single-particle trajectories reveal two-state diffusion-kinetics of hOGG1 proteins on DNA
Abstract
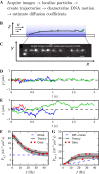
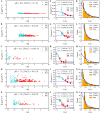
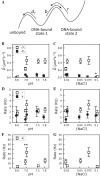
We reanalyze trajectories of hOGG1 repair proteins diffusing on DNA. A previous analysis of these trajectories with the popular mean-squared-displacement approach revealed only simple diffusion. Here, a new optimal estimator of diffusion coefficients reveals two-state kinetics of the protein. A simple, solvable model, in which the protein randomly switches between a loosely bound, highly mobile state and a tightly bound, less mobile state is the simplest possible dynamic model consistent with the data. It yields accurate estimates of hOGG1's (i) diffusivity in each state, uncorrupted by experimental errors arising from shot noise, motion blur and thermal fluctuations of the DNA; (ii) rates of switching between states and (iii) rate of detachment from the DNA. The protein spends roughly equal time in each state. It detaches only from the loosely bound state, with a rate that depends on pH and the salt concentration in solution, while its rates for switching between states are insensitive to both. The diffusivity in the loosely bound state depends primarily on pH and is three to ten times higher than in the tightly bound state. We propose and discuss some new experiments that take full advantage of the new tools of analysis presented here.
Figures

References
-
- Brameshuber M., Schütz G.J.. Detection and quantification of biomolecular association in living cells using single-molecule microscopy. Methods Enzymol. 2012; 505:159–186. - PubMed
-
- Alberts B., Bray D., Lewis J., Raff M., Roberts K., Watson J.D.. Molecular Biology of the Cell. 1994; 3rd ednGarland Publishing, Inc; 95–563.
-
- van Loenhout M.T.J., de Grunt M.V., Dekker C.. Dynamics of DNA supercoils. Science. 2012; 338:94–97. - PubMed
-
- Wang Y.M., Austin R.H., Cox E.C.. Single molecule measurements of repressor protein 1D diffusion on DNA. Phys. Rev. Lett. 2006; 97:048302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

