Acute ethanol exposure has bidirectional actions on the endogenous neuromodulator adenosine in rat hippocampus
- PMID: 29361192
- PMCID: PMC5901169
- DOI: 10.1111/bph.14152
Acute ethanol exposure has bidirectional actions on the endogenous neuromodulator adenosine in rat hippocampus
Abstract
Background and purpose: Ethanol is a widely used recreational drug with complex effects on physiological and pathological brain function. In epileptic patients, the use of ethanol can modify seizure initiation and subsequent seizure activity with reports of ethanol being both pro- and anticonvulsant. One proposed target of ethanol's actions is the neuromodulator adenosine, which is released during epileptic seizures to feedback and inhibit the occurrence of subsequent seizures. Here, we investigated the actions of acute ethanol exposure on adenosine signalling in rat hippocampus.
Experimental approach: We have combined electrophysiology with direct measurements of extracellular adenosine using microelectrode biosensors in rat hippocampal slices.
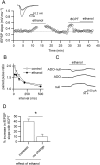
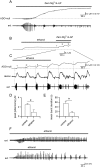
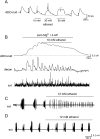
Key results: We found that ethanol has bidirectional actions on adenosine signalling: depressant concentrations of ethanol (50 mM) increased the basal extracellular concentration of adenosine under baseline conditions, leading to the inhibition of synaptic transmission, but it inhibited adenosine release during evoked seizure activity in brain slices. The reduction in activity-dependent adenosine release was in part produced by effects on NMDA receptors, although other mechanisms also appeared to be involved. Low concentrations of ethanol (10-15 mM) enhanced pathological network activity by selectively blocking activity-dependent adenosine release.
Conclusions and implications: The complex dose-dependent actions of ethanol on adenosine signalling could in part explain the mixture of pro-convulsant and anticonvulsant actions of ethanol that have previously been reported.
© 2018 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures





Similar articles
-
Endogenous adenosine modulates epileptiform activity in rat hippocampus in a receptor subtype-dependent manner.Eur J Neurosci. 2004 May;19(9):2539-50. doi: 10.1111/j.0953-816X.2004.03355.x. Eur J Neurosci. 2004. PMID: 15128407
-
Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus.Neuropharmacology. 2009 Feb;56(2):429-37. doi: 10.1016/j.neuropharm.2008.09.016. Epub 2008 Oct 10. Neuropharmacology. 2009. PMID: 18957298 Free PMC article.
-
The combination of ribose and adenine promotes adenosine release and attenuates the intensity and frequency of epileptiform activity in hippocampal slices: Evidence for the rapid depletion of cellular ATP during electrographic seizures.J Neurochem. 2018 Oct;147(2):178-189. doi: 10.1111/jnc.14543. Epub 2018 Sep 10. J Neurochem. 2018. PMID: 29964329 Free PMC article.
-
High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices.J Neurochem. 2003 Sep;86(6):1506-15. doi: 10.1046/j.1471-4159.2003.01957.x. J Neurochem. 2003. PMID: 12950459
-
Interactions between ethanol, endogenous adenosine and adenosine uptake in hippocampal brain slices.J Pharmacol Exp Ther. 1996 Aug;278(2):542-6. J Pharmacol Exp Ther. 1996. PMID: 8768702
Cited by
-
Adenosine-Related Mechanisms in Non-Adenosine Receptor Drugs.Cells. 2020 Apr 13;9(4):956. doi: 10.3390/cells9040956. Cells. 2020. PMID: 32295065 Free PMC article. Review.
-
Alcohol Use Disorder: Neurobiology and Therapeutics.Biomedicines. 2022 May 21;10(5):1192. doi: 10.3390/biomedicines10051192. Biomedicines. 2022. PMID: 35625928 Free PMC article. Review.
-
Inhibition of ecto-5'-nucleotidase and adenosine deaminase is able to reverse long-term behavioural effects of early ethanol exposure in zebrafish (Danio rerio).Sci Rep. 2020 Oct 20;10(1):17809. doi: 10.1038/s41598-020-74832-0. Sci Rep. 2020. PMID: 33082435 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

