Bespoke Pretargeted Nanoradioimmunotherapy for the Treatment of Non-Hodgkin Lymphoma
- PMID: 29361211
- PMCID: PMC6713228
- DOI: 10.1021/acsnano.7b08122
Bespoke Pretargeted Nanoradioimmunotherapy for the Treatment of Non-Hodgkin Lymphoma
Erratum in
-
Correction to "Bespoke Pretargeted Nanoradioimmunotherapy for the Treatment of Non-Hodgkin Lymphoma".ACS Nano. 2024 Jun 11;18(23):15326. doi: 10.1021/acsnano.4c05757. Epub 2024 May 28. ACS Nano. 2024. PMID: 38806286 No abstract available.
Abstract
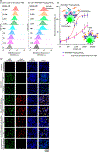
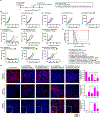
Non-Hodgkin lymphoma (NHL) is one of the most common types of hematologic malignancies. Pretargeted radioimmunotherapy (PRIT), the sequential administration of a bispecific antibody-based primary tumor-targeting component followed by a radionucleotide-labeled treatment effector, has been developed to improve the treatment efficacy and to reduce the side effects of conventional RIT. Despite the preclinical success of PRIT, clinical trials revealed that the immunogenicity of the bispecific antibody as well as the presence of competing endogenous effector molecules often compromised the treatment. One strategy to improve PRIT is to utilize bio-orthogonal ligation reactions to minimize immunogenicity and improve targeting. Herein, we report a translatable pretargeted nanoradioimmunotherapy strategy for the treatment of NHL. This pretargeting system is composed of a dibenzylcyclooctyne (DBCO)-functionalized anti-CD20 antibody (α-CD20) tumor-targeting component and an azide- and yttrium-90-(90Y) dual-functionalized dendrimer. The physicochemical properties of both pretargeting components have been extensively studied. We demonstrated that an optimized dual-functionalized dendrimer can undergo rapid strain-promoted azide-alkyne cycloaddition with the DBCO-functionalized α-CD20 at the physiological conditions. The treatment effector in our pretargeting system can not only selectively deliver radionucleotides to the target tumor cells but also increase the complement-dependent cytotoxicity of α-CD20 and thus enhance the antitumor effects, as justified by comprehensive in vitro and in vivo studies in mouse NHL xenograft and disseminated models.
Keywords: antibody; bioorthogonal ligation reaction; dendrimer; non-Hodgkin lymphoma; pretargeted radioimmunotherapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Comparative efficacy of 177Lu and 90Y for anti-CD20 pretargeted radioimmunotherapy in murine lymphoma xenograft models.PLoS One. 2015 Mar 18;10(3):e0120561. doi: 10.1371/journal.pone.0120561. eCollection 2015. PLoS One. 2015. PMID: 25785845 Free PMC article.
-
Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin's lymphoma (NHL).Crit Rev Oncol Hematol. 2001 Oct;40(1):37-51. doi: 10.1016/s1040-8428(01)00133-0. Crit Rev Oncol Hematol. 2001. PMID: 11578915 Clinical Trial.
-
Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin's lymphoma (NHL): initial phase I/II study results.Cancer Biother Radiopharm. 2000 Feb;15(1):15-29. doi: 10.1089/cbr.2000.15.15. Cancer Biother Radiopharm. 2000. PMID: 10740649 Clinical Trial.
-
[Radioimmunotherapy for non-Hodgkin lymphoma: historical development and current status].Rev Esp Med Nucl. 2006 Jan-Feb;25(1):42-54. doi: 10.1157/13083351. Rev Esp Med Nucl. 2006. PMID: 16540013 Review. Spanish.
-
[Radioimmunotherapy with 90Y-ibritumomab tiuxetan in lymphomas].Rev Esp Med Nucl. 2006 Jan-Feb;25(1):55-68; quiz 69-70.. doi: 10.1157/13083353. Rev Esp Med Nucl. 2006. PMID: 16540015 Review. Spanish. No abstract available.
Cited by
-
Nanotechnology in Radiation Oncology.Hematol Oncol Clin North Am. 2019 Dec;33(6):1071-1093. doi: 10.1016/j.hoc.2019.08.002. Epub 2019 Oct 1. Hematol Oncol Clin North Am. 2019. PMID: 31668207 Free PMC article. Review.
-
Drug delivery methods for cancer immunotherapy.Drug Deliv Transl Res. 2024 Jan;14(1):30-61. doi: 10.1007/s13346-023-01405-9. Epub 2023 Aug 16. Drug Deliv Transl Res. 2024. PMID: 37587290 Free PMC article. Review.
-
Pretargeted delivery of PI3K/mTOR small-molecule inhibitor-loaded nanoparticles for treatment of non-Hodgkin's lymphoma.Sci Adv. 2020 Apr 1;6(14):eaaz9798. doi: 10.1126/sciadv.aaz9798. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32270047 Free PMC article.
-
Nanoparticles and bioorthogonal chemistry joining forces for improved biomedical applications.Nanoscale Adv. 2021 Jan 21;3(5):1261-1292. doi: 10.1039/d0na00873g. eCollection 2021 Mar 9. Nanoscale Adv. 2021. PMID: 36132873 Free PMC article. Review.
-
Radiotheranostic Agents in Hematological Malignancies.Front Immunol. 2022 Jul 5;13:911080. doi: 10.3389/fimmu.2022.911080. eCollection 2022. Front Immunol. 2022. PMID: 35865548 Free PMC article. Review.
References
-
- Siegel RL; Miller KD; Jemal A Cancer Statistics, 2017. Ca-Cancer J. Clin 2017, 67, 7–30. - PubMed
-
- Coiffier B; Lepage E; Briere J; Herbrecht R; Tilly H; Bouabdallah R; Morel P; Van Den Neste E; Salles G; Gaulard P; Reyes F; Lederlin P; Gisselbrecht C CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med 2002, 346, 235–42. - PubMed
-
- Feugier P; Van Hoof A; Sebban C; Solal-Celigny P; Bouabdallah R; Ferme C; Christian B; Lepage E; Tilly H; Morschhauser F; Gaulard P; Salles G; Bosly A; Gisselbrecht C; Reyes F; Coiffier B Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol 2005, 23, 4117–26. - PubMed
-
- Molina A A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu. Rev. Med 2008, 59, 237–50. - PubMed
-
- Boye J; Elter T; Engert A An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann. Oncol 2003, 14, 520–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

