Progress in the Development of Preventative Drugs for Cisplatin-Induced Hearing Loss
- PMID: 29361217
- PMCID: PMC6043375
- DOI: 10.1021/acs.jmedchem.7b01653
Progress in the Development of Preventative Drugs for Cisplatin-Induced Hearing Loss
Abstract
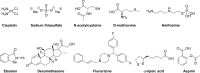
Cisplatin is a highly effective treatment for malignant cancers and has become a cornerstone in chemotherapeutic regimens. Unfortunately, its use in the clinic is often coupled with a high incidence of severe hearing loss. Over the past few decades, enormous effort has been put forth to find protective agents that selectively protect against the ototoxic side effects of cisplatin and do not interfere with its antitumoral activity. Many therapies have been successful in preclinical work, but only a few have shown any protection in the clinic, and none have been approved by the FDA. This review summarizes the clinical and preclinical studies of the most effective small-molecule candidates currently in clinical trials, while also detailing their molecular mechanisms of action, to gain insight for future drug development in the field.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

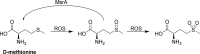

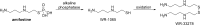
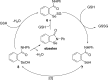

References
-
- Greene J. B.; Standring R.; Siddiqui F.; Ahsan S. F. Incidence of cisplatin induced ototoxicity in adults with head and neck cancer. Adv. Otolaryngol. 2015, 56, 1–4. 10.1155/2015/245613. - DOI
-
- Freyer D. R.; Chen L.; Krailo M. D.; Knight K.; Villaluna D.; Bliss B.; Pollock B. H.; Ramdas J.; Lange B.; Van Hoff D.; VanSoelen M. L.; Wiernikowski J.; Neuwelt E. A.; Sung L. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 63–74. 10.1016/S1470-2045(16)30625-8. - DOI - PMC - PubMed
-
- Brock P. R.; Childs M.; Rajput K.; Maibach R.; Roebuck D.; Sullivan M. J.; Laithier V.; Ronghe M.; dall’Igna P.; Hiyama E.; Brichard B.; Skeen J.; Mateos M. E.; Fabre M.; Perilongo G.; Czauderna P.; Morland B.; Neuwelt E. A. Two-year results of clinical efficacy of cisplatin in combination with sodium thiosulfate (STS) vs cisplatin alone in a randomized phase III trial for standard risk hepatoblastoma (SR-HB): SIOPEL 6. J. Clin. Oncol. 2016, 34, 10514–10514. 10.1200/JCO.2016.34.15_suppl.10514. - DOI
-
- Zuur C. L.; Simis Y. J.; Lansdaal P. E.; Hart A. A.; Schornagel J. H.; Dreschler W. A.; Rasch C. R.; Balm A. J. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J. Clin. Oncol. 2007, 25, 3759–3765. 10.1200/JCO.2006.08.9540. - DOI - PubMed
-
- Orgel E.NAC to Prevent Cisplatin-induced Hearing Loss, 2014. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02094625. (accessed Nov 6, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

