Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression
- PMID: 29361501
- PMCID: PMC5835545
- DOI: 10.1016/j.ebiom.2017.12.021
Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression
Abstract
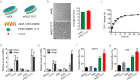
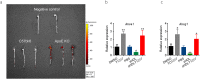
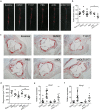
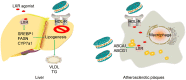
Targeting at enhancing reverse cholesterol transport (RCT) is apromising strategy for treating atherosclerosis via infusion of reconstitute high density lipoprotein (HDL) as cholesterol acceptors or increase of cholesterol efflux by activation of macrophage liver X receptors (LXRs). However, systemic activation of LXRs triggers excessive lipogenesis in the liver and infusion of HDL downregulates cholesterol efflux from macrophages. Here we describe an enlightened strategy using phospholipid reconstituted apoA-I peptide (22A)-derived synthetic HDL (sHDL) to deliver LXR agonists to the atheroma and examine their effect on atherosclerosis regression in vivo. A synthetic LXR agonist, T0901317 (T1317) was encapsulated in sHDL nanoparticles (sHDL-T1317). Similar to the T1317 compound, the sHDL-T1317 nanoparticles upregulated the expression of ATP-binding cassette transporters and increased cholesterol efflux in macrophages in vitro and in vivo. The sHDL nanoparticles accumulated in the atherosclerotic plaques of ApoE-deficient mice. Moreover, a 6-week low-dose LXR agonist-sHDL treatment induced atherosclerosis regression while avoiding lipid accumulation in the liver. These findings identify LXR agonist loaded sHDL nanoparticles as a promising therapeutic approach to treat atherosclerosis by targeting RCT in a multifaceted manner: sHDL itself serving as both a drug carrier and cholesterol acceptor and the LXR agonist mediating upregulation of ABC transporters in the aorta.
Keywords: Atherosclerosis; High-density lipoprotein; Liver X receptors; Macrophage; Reverse cholesterol transport.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Comment in
-
LXRs are finally being adequately targeted in atherosclerosis.Ann Transl Med. 2018 Nov;6(Suppl 1):S28. doi: 10.21037/atm.2018.09.35. Ann Transl Med. 2018. PMID: 30613603 Free PMC article. No abstract available.
-
Treating atherosclerosis: targeting risk factors should not be the only option.Ann Transl Med. 2018 Nov;6(Suppl 1):S34. doi: 10.21037/atm.2018.09.40. Ann Transl Med. 2018. PMID: 30613609 Free PMC article. No abstract available.
References
-
- Adorni M.P., Zimetti F., Billheimer J.T., Wang N., Rader D.J., Phillips M.C., Rothblat G.H. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 2007;48:2453–2462. - PubMed
-
- Aiello R.J., Brees D., Bourassa P.A., Royer L., Lindsey S., Coskran T., Haghpassand M., Francone O.L. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 2002;22:630–637. - PubMed
-
- Albrecht C., Soumian S., Amey J.S., Sardini A., Higgins C.F., Davies A.H., Gibbs R.G. ABCA1 expression in carotid atherosclerotic plaques. Stroke. 2004;35:2801–2806. - PubMed
-
- Belalcazar L.M., Merched A., Carr B., Oka K., Chen K.H., Pastore L., Beaudet A., Chan L. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. - PubMed
-
- Bochem A.E., VAN Wijk D.F., Holleboom A.G., Duivenvoorden R., Motazacker M.M., Dallinga-Thie G.M., DE Groot E., Kastelein J.J., Nederveen A.J., Hovingh G.K., Stroes E.S. ABCA1 mutation carriers with low high-density lipoprotein cholesterol are characterized by a larger atherosclerotic burden. Eur. Heart J. 2013;34:286–291. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

