Inorganic arsenic causes fatty liver and interacts with ethanol to cause alcoholic liver disease in zebrafish
- PMID: 29361514
- PMCID: PMC5894941
- DOI: 10.1242/dmm.031575
Inorganic arsenic causes fatty liver and interacts with ethanol to cause alcoholic liver disease in zebrafish
Abstract
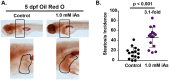
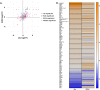
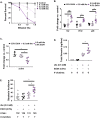
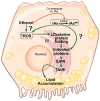
The rapid increase in fatty liver disease (FLD) incidence is attributed largely to genetic and lifestyle factors; however, environmental toxicants are a frequently overlooked factor that can modify the effects of more common causes of FLD. Chronic exposure to inorganic arsenic (iAs) is associated with liver disease in humans and animal models, but neither the mechanism of action nor the combinatorial interaction with other disease-causing factors has been fully investigated. Here, we examined the contribution of iAs to FLD using zebrafish and tested the interaction with ethanol to cause alcoholic liver disease (ALD). We report that zebrafish exposed to iAs throughout development developed specific phenotypes beginning at 4 days post-fertilization (dpf), including the development of FLD in over 50% of larvae by 5 dpf. Comparative transcriptomic analysis of livers from larvae exposed to either iAs or ethanol revealed the oxidative stress response and the unfolded protein response (UPR) caused by endoplasmic reticulum (ER) stress as common pathways in both these models of FLD, suggesting that they target similar cellular processes. This was confirmed by our finding that arsenic is synthetically lethal with both ethanol and a well-characterized ER-stress-inducing agent (tunicamycin), suggesting that these exposures work together through UPR activation to cause iAs toxicity. Most significantly, combined exposure to sub-toxic concentrations of iAs and ethanol potentiated the expression of UPR-associated genes, cooperated to induce FLD, reduced the expression of as3mt, which encodes an arsenic-metabolizing enzyme, and significantly increased the concentration of iAs in the liver. This demonstrates that iAs exposure is sufficient to cause FLD and that low doses of iAs can potentiate the effects of ethanol to cause liver disease.This article has an associated First Person interview with the first author of the paper.
Keywords: Arsenic; Environmental exposure; Ethanol; Fatty liver disease.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Ahsan H., Chen Y., Parvez F., Argos M., Hussain A. I., Momotaj H., Levy D., Van Geen A., Howe G. and Graziano J. (2006). Health effects of arsenic longitudinal study (heals): Description of a multidisciplinary epidemiologic investigation. J. Expo. Sci. Environ. Epidemiol. 16, 191-205. 10.1038/sj.jea.7500449 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

