Divergent effects of adrenaline in human induced pluripotent stem cell-derived cardiomyocytes obtained from hypertrophic cardiomyopathy
- PMID: 29361520
- PMCID: PMC5894949
- DOI: 10.1242/dmm.032896
Divergent effects of adrenaline in human induced pluripotent stem cell-derived cardiomyocytes obtained from hypertrophic cardiomyopathy
Abstract
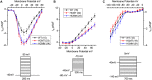
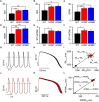
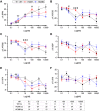
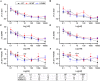
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiac disease that affects the heart muscle with diverse clinical outcomes. HCM can cause sudden cardiac death (SCD) during or immediately after mild to rigorous physical activity in young patients. However, the mechanism causing SCD as a result of exercise remains unknown, but exercise-induced ventricular arrhythmias are thought to be responsible for this fatal consequence. To understand the disease mechanism behind HCM in a better way, we generated patient-specific induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) from HCM patients carrying either the MYBPC3-Gln1061X or TPM1-Asp175Asn mutation. We extensively investigated the effects of low to high concentrations of adrenaline on action potential characteristics, and the occurrence of arrhythmias in the presence of various concentrations of adrenaline and in wash-out condition. We classified and quantified different types of arrhythmias observed in hiPSC-CMs, and found that the occurrence of arrhythmias was dependent on concentrations of adrenaline and positions of mutations in genes causing HCM. In addition, we observed ventricular tachycardia types of arrhythmias in hiPSC-CMs carrying the TPM1-Asp175Asn mutation. We additionally examined the antiarrhythmic potency of bisoprolol in HCM-specific hiPSC-CMs. However, bisoprolol could not reduce the occurrence of arrhythmias during administration or during the wash-out condition of adrenaline in HCM-specific hiPSC-CMs. Our study demonstrates hiPSC-CMs as a promising tool for studying HCM. The experimental design used in this study could be suitable and beneficial for studying other components and drugs related to cardiac disease in general.
Keywords: Adrenaline; Arrhythmia; Bisoprolol; HCM; hiPSC-CMs.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Mutation-Specific Phenotypes in hiPSC-Derived Cardiomyocytes Carrying Either Myosin-Binding Protein C Or α-Tropomyosin Mutation for Hypertrophic Cardiomyopathy.Stem Cells Int. 2016;2016:1684792. doi: 10.1155/2016/1684792. Epub 2015 Dec 28. Stem Cells Int. 2016. PMID: 27057166 Free PMC article.
-
Isogenic Pairs of hiPSC-CMs with Hypertrophic Cardiomyopathy/LVNC-Associated ACTC1 E99K Mutation Unveil Differential Functional Deficits.Stem Cell Reports. 2018 Nov 13;11(5):1226-1243. doi: 10.1016/j.stemcr.2018.10.006. Epub 2018 Nov 1. Stem Cell Reports. 2018. PMID: 30392975 Free PMC article.
-
Hypertrophic cardiomyopathy dysfunction mimicked in human engineered heart tissue and improved by sodium-glucose cotransporter 2 inhibitors.Cardiovasc Res. 2024 Mar 14;120(3):301-317. doi: 10.1093/cvr/cvae004. Cardiovasc Res. 2024. PMID: 38240646 Free PMC article.
-
Abnormalities in sodium current and calcium homoeostasis as drivers of arrhythmogenesis in hypertrophic cardiomyopathy.Cardiovasc Res. 2020 Jul 15;116(9):1585-1599. doi: 10.1093/cvr/cvaa124. Cardiovasc Res. 2020. PMID: 32365196 Review.
-
Advances in Hypertrophic Cardiomyopathy Disease Modelling Using hiPSC-Derived Cardiomyocytes.Can J Cardiol. 2024 May;40(5):766-776. doi: 10.1016/j.cjca.2023.11.009. Epub 2023 Nov 10. Can J Cardiol. 2024. PMID: 37952715 Review.
Cited by
-
INDUCED PLURIPOTENT STEM CELLS FOR MODELLING ENERGETIC ALTERATIONS IN HYPERTROPHIC CARDIOMYOPATHY.Cond Med. 2019;2(4):142-151. Cond Med. 2019. PMID: 32457935 Free PMC article.
-
Seamless integration of CMOS microsensors into open microfluidic systems.Lab Chip. 2025 Apr 29;25(9):2205-2221. doi: 10.1039/d4lc01000k. Lab Chip. 2025. PMID: 40171768 Free PMC article.
-
The Junctophilin-2 Mutation p.(Thr161Lys) Is Associated with Hypertrophic Cardiomyopathy Using Patient-Specific iPS Cardiomyocytes and Demonstrates Prolonged Action Potential and Increased Arrhythmogenicity.Biomedicines. 2023 May 27;11(6):1558. doi: 10.3390/biomedicines11061558. Biomedicines. 2023. PMID: 37371654 Free PMC article.
-
Generation of induced pluripotent stem cells from an individual with early onset and severe hypertrophic cardiomyopathy linked to MYBPC3: c.772G > A mutation.Hum Cell. 2024 Jul;37(4):1205-1214. doi: 10.1007/s13577-024-01073-y. Epub 2024 May 18. Hum Cell. 2024. PMID: 38762696 Free PMC article.
-
Uncovering Inherited Cardiomyopathy With Human Induced Pluripotent Stem Cells.Front Cell Dev Biol. 2021 May 17;9:672039. doi: 10.3389/fcell.2021.672039. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34079803 Free PMC article. Review.
References
-
- Barajas-Martínez H., Hu D., Goodrow R. J., Joyce F. and Antzelevitch C. (2013). Electrophysiologic characteristics and pharmacologic response of human cardiomyocytes isolated from a patient with hypertrophic cardiomyopathy. Pacing Clin. Electrophysiol. 36, 1512-1515. 10.1111/pace.12227 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

