Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast
- PMID: 29361568
- PMCID: PMC5818005
- DOI: 10.1242/dev.158501
Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast
Erratum in
-
Correction: Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast (doi: 10.1242/dev.158501).Development. 2018 Aug 1;145(15):dev169672. doi: 10.1242/dev.169672. Development. 2018. PMID: 30068690 Free PMC article. No abstract available.
Abstract
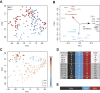
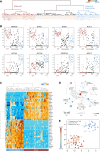
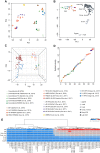
Single-cell profiling techniques create opportunities to delineate cell fate progression in mammalian development. Recent studies have provided transcriptome data from human pre-implantation embryos, in total comprising nearly 2000 individual cells. Interpretation of these data is confounded by biological factors, such as variable embryo staging and cell-type ambiguity, as well as technical challenges in the collective analysis of datasets produced with different sample preparation and sequencing protocols. Here, we address these issues to assemble a complete gene expression time course spanning human pre-implantation embryogenesis. We identify key transcriptional features over developmental time and elucidate lineage-specific regulatory networks. We resolve post-hoc cell-type assignment in the blastocyst, and define robust transcriptional prototypes that capture epiblast and primitive endoderm lineages. Examination of human pluripotent stem cell transcriptomes in this framework identifies culture conditions that sustain a naïve state pertaining to the inner cell mass. Our approach thus clarifies understanding both of lineage segregation in the early human embryo and of in vitro stem cell identity, and provides an analytical resource for comparative molecular embryology.
Keywords: Embryo; Human; Pluripotency; RNA-seq; Single cell.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsG.G. and A.S. are inventors on a patent filing by the University of Cambridge relating to human naïve pluripotent stem cells.
Figures




References
-
- Boroviak T., Loos R., Lombard P., Okahara J., Behr R., Sasaki E., Nichols J., Smith A. and Bertone P. (2015). Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev. Cell 35, 366-382. 10.1016/j.devcel.2015.10.011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

