miR-9a mediates the role of Lethal giant larvae as an epithelial growth inhibitor in Drosophila
- PMID: 29361610
- PMCID: PMC5829493
- DOI: 10.1242/bio.027391
miR-9a mediates the role of Lethal giant larvae as an epithelial growth inhibitor in Drosophila
Abstract
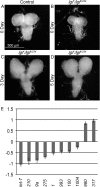
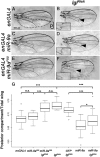
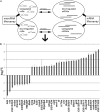
Drosophila lethal giant larvae (lgl) encodes a conserved tumor suppressor with established roles in cell polarity, asymmetric division, and proliferation control. Lgl's human orthologs, HUGL1 and HUGL2, are altered in human cancers, however, its mechanistic role as a tumor suppressor remains poorly understood. Based on a previously established connection between Lgl and Fragile X protein (FMRP), a miRNA-associated translational regulator, we hypothesized that Lgl may exert its role as a tumor suppressor by interacting with the miRNA pathway. Consistent with this model, we found that lgl is a dominant modifier of Argonaute1 overexpression in the eye neuroepithelium. Using microarray profiling we identified a core set of ten miRNAs that are altered throughout tumorigenesis in Drosophila lgl mutants. Among these are several miRNAs previously linked to human cancers including miR-9a, which we found to be downregulated in lgl neuroepithelial tissues. To determine whether miR-9a can act as an effector of Lgl in vivo, we overexpressed it in the context of lgl knock-down by RNAi and found it able to reduce the overgrowth phenotype caused by Lgl loss in epithelia. Furthermore, cross-comparisons between miRNA and mRNA profiling in lgl mutant tissues and human breast cancer cells identified thrombospondin (tsp) as a common factor altered in both fly and human breast cancer tumorigenesis models. Our work provides the first evidence of a functional connection between Lgl and the miRNA pathway, demonstrates that miR-9a mediates Lgl's role in restricting epithelial proliferation, and provides novel insights into pathways controlled by Lgl during tumor progression.
Keywords: Drosophila; Epithelial growth; miRNA.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Abu-Safieh L., Abboud E. B., Alkuraya H., Shamseldin H., Al-Enzi S., Al-Abdi L., Hashem M., Colak D., Jarallah A., Ahmad H. et al. (2011). Mutation of IGFBP7 causes upregulation of BRAF/MEK/ERK pathway and familial retinal arterial macroaneurysms. Am. J. Hum. Genet. 89, 313-319. 10.1016/j.ajhg.2011.07.010 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

