Route and Type of Formulation Administered Influences the Absorption and Disposition of Vitamin B12 Levels in Serum
- PMID: 29361736
- PMCID: PMC5872098
- DOI: 10.3390/jfb9010012
Route and Type of Formulation Administered Influences the Absorption and Disposition of Vitamin B12 Levels in Serum
Abstract
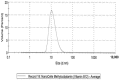
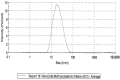
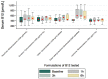
The administration of biological compounds that optimize health benefits is an ever-evolving therapeutic goal. Pharmaceutical and other adjunctive biological compounds have been administered via many different routes in order to produce a systemic pharmacological effect. The article summarizes the findings from an Australian comparative study in adults administered vitamin B12 through different oral delivery platforms. A total of 16 subjects (9 males, 7 females) voluntarily partook in a comparative clinical study of five different vitamin B12 formulations across a six-month period, completing 474 person-hours of cumulative contribution, that was equivalent to an n = 60 participation. A nanoparticle delivered vitamin B12 through a NanoCelle platform was observed to be significantly (p < 0.05) better absorbed than all other dose equivalent platforms (i.e., tablets, emulsions, or liposomes) from baseline to 1, 3, and 6 h of the study period. The nanoparticle platform delivered vitamin B12 demonstrated an enhanced and significant absorption profile as exemplified by rapid systemic detection (i.e., 1 h from baseline) when administered to the oro-buccal mucosa with no reports of any adverse events of toxicity. The nanoparticle formulation of methylcobalamin (1000 µg/dose in 0.3 mL volume) showed bioequivalence only with a chewable-dissolvable tablet that administered a five times higher dose of methylcobalamin (5000 µg) per tablet. This study has demonstrated that an active metabolite embedded in a functional biomaterial (NanoCelle) may constitute a drug delivery method that can better access the circulatory system.
Keywords: NanoCelle; cyanocobalamin; emulsion; liposome; methylcobalamin; nanoparticles; tablets; vitamin B12.
Conflict of interest statement
L.V., J.Z., R.M., S.D.F., S.H., and D.R. participate in Medlab Clinical’s nanoparticle delivery technology research. The authors have no further conflicts of interest relevant to the content of this manuscript.
Figures
References
-
- Kwan K.C. Oral bioavailability and first-pass effects. Drug Metab. Dispos. 1997;25:1329–1336. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources