Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels
- PMID: 29361737
- PMCID: PMC5796258
- DOI: 10.3390/ijms19010315
Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels
Abstract
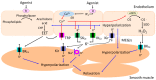
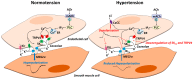
Upon stimulation with agonists and shear stress, the vascular endothelium of different vessels selectively releases several vasodilator factors such as nitric oxide and prostacyclin. In addition, vascular endothelial cells of many vessels regulate the contractility of the vascular smooth muscle cells through the generation of endothelium-dependent hyperpolarization (EDH). There is a general consensus that the opening of small- and intermediate-conductance Ca2+-activated K⁺ channels (SKCa and IKCa) is the initial mechanistic step for the generation of EDH. In animal models and humans, EDH and EDH-mediated relaxations are impaired during hypertension, and anti-hypertensive treatments restore such impairments. However, the underlying mechanisms of reduced EDH and its improvement by lowering blood pressure are poorly understood. Emerging evidence suggests that alterations of endothelial ion channels such as SKCa channels, inward rectifier K⁺ channels, Ca2+-activated Cl- channels, and transient receptor potential vanilloid type 4 channels contribute to the impaired EDH during hypertension. In this review, we attempt to summarize the accumulating evidence regarding the pathophysiological role of endothelial ion channels, focusing on their relationship with EDH during hypertension.
Keywords: Ca2+-activated Cl− channel; Ca2+-activated K+ channel; endothelial function; endothelium-dependent hyperpolarization; endothelium-derived hyperpolarizing factor; hypertension; transient receptor potential vanilloid type 4 channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Downregulation of Endothelial Transient Receptor Potential Vanilloid Type 4 Channel and Small-Conductance of Ca2+-Activated K+ Channels Underpins Impaired Endothelium-Dependent Hyperpolarization in Hypertension.Hypertension. 2017 Jan;69(1):143-153. doi: 10.1161/HYPERTENSIONAHA.116.07110. Epub 2016 Nov 21. Hypertension. 2017. PMID: 27872234
-
Endothelium-dependent hyperpolarization: age, gender and blood pressure, do they matter?Acta Physiol (Oxf). 2017 Jan;219(1):108-123. doi: 10.1111/apha.12628. Epub 2015 Dec 1. Acta Physiol (Oxf). 2017. PMID: 26548576 Review.
-
Increased inward rectifier K+ current of coronary artery smooth muscle cells in spontaneously hypertensive rats; partial compensation of the attenuated endothelium-dependent relaxation via Ca2+ -activated K+ channels.Clin Exp Pharmacol Physiol. 2020 Jan;47(1):38-48. doi: 10.1111/1440-1681.13168. Epub 2019 Aug 24. Clin Exp Pharmacol Physiol. 2020. PMID: 31444788
-
Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease.Clin Exp Pharmacol Physiol. 2004 Sep;31(9):641-9. doi: 10.1111/j.1440-1681.2004.04053.x. Clin Exp Pharmacol Physiol. 2004. PMID: 15479173 Review.
-
Acid-sensing ion channel 1a activates IKCa/SKCa channels and contributes to endothelium-dependent dilation.J Gen Physiol. 2023 Feb 6;155(2):e202213173. doi: 10.1085/jgp.202213173. Epub 2022 Dec 9. J Gen Physiol. 2023. PMID: 36484717 Free PMC article.
Cited by
-
Vascular Endothelial Cell Biology: An Update.Int J Mol Sci. 2019 Sep 7;20(18):4411. doi: 10.3390/ijms20184411. Int J Mol Sci. 2019. PMID: 31500313 Free PMC article. Review.
-
Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide.Biomedicines. 2024 Dec 15;12(12):2851. doi: 10.3390/biomedicines12122851. Biomedicines. 2024. PMID: 39767758 Free PMC article. Review.
-
Chloride Ions, Vascular Function and Hypertension.Biomedicines. 2022 Sep 18;10(9):2316. doi: 10.3390/biomedicines10092316. Biomedicines. 2022. PMID: 36140417 Free PMC article. Review.
-
Internalization and Transportation of Endothelial Cell Surface KCa2.3 and KCa3.1 in Normal Pregnancy and Preeclampsia.Oxid Med Cell Longev. 2019 Nov 23;2019:5820839. doi: 10.1155/2019/5820839. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31871552 Free PMC article.
-
Oxidative Stress-Induced Hypertension of Developmental Origins: Preventive Aspects of Antioxidant Therapy.Antioxidants (Basel). 2022 Mar 7;11(3):511. doi: 10.3390/antiox11030511. Antioxidants (Basel). 2022. PMID: 35326161 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

