Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis
- PMID: 29361749
- PMCID: PMC5796268
- DOI: 10.3390/ijms19010326
Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis
Abstract
Background: In rheumatoid arthritis (RA), anti-citrullinated protein/peptide antibodies (ACPAs) are responsible for disease onset and progression, however, our knowledge is limited on ligand binding affinities of autoantibodies with different citrulline-peptide specificity.
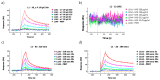
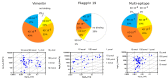
Methods: Citrulline-peptide-specific ACPA IgGs were affinity purified and tested by ELISA. Binding affinities of ACPA IgGs and serum antibodies were compared by surface plasmon resonance (SPR) analysis. Bifunctional nanoparticles harboring a multi-epitope citrulline-peptide and a complement-activating peptide were used to induce selective depletion of ACPA-producing B cells.
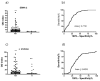
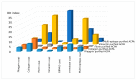
Results: KD values of affinity-purified ACPA IgGs varied between 10-6 and 10-8 M and inversely correlated with disease activity. Based on their cross-reaction with citrulline-peptides, we designed a novel multi-epitope peptide, containing Cit-Gly and Ala-Cit motifs in two-two copies, separated with a short, neutral spacer. This peptide detected antibodies in RA sera with 66% sensitivity and 98% specificity in ELISA and was recognized by 90% of RA sera, while none of the healthy samples in SPR. When coupled to nanoparticles, the multi-epitope peptide specifically targeted and depleted ACPA-producing B cells ex vivo.
Conclusions: The unique multi-epitope peptide designed based on ACPA cross-reactivity might be suitable to develop better diagnostics and novel therapies for RA.
Keywords: affinity; autoantibody; citrulline-peptides; cross-reaction; rheumatoid arthritis; targeting.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
Recognition of new citrulline-containing peptide epitopes by autoantibodies produced in vivo and in vitro by B cells of rheumatoid arthritis patients.Immunology. 2014 Feb;141(2):181-91. doi: 10.1111/imm.12175. Immunology. 2014. PMID: 24116744 Free PMC article.
-
Antibody responses to de novo identified citrullinated fibrinogen peptides in rheumatoid arthritis and visualization of the corresponding B cells.Arthritis Res Ther. 2016 Dec 1;18(1):284. doi: 10.1186/s13075-016-1181-0. Arthritis Res Ther. 2016. PMID: 27906052 Free PMC article.
-
Recognition of Glycine Versus Nonglycine Citrulline Motifs Dictating the HLA Class II Association of Anticitrullinated Protein Antibodies: Insights From Autoantibody Profiling of 6,900 Scandinavian Patients With Rheumatoid Arthritis.Arthritis Rheumatol. 2025 Sep;77(9):1179-1193. doi: 10.1002/art.43161. Epub 2025 Apr 30. Arthritis Rheumatol. 2025. PMID: 40116570 Free PMC article.
-
Anti-citrullinated peptides as autoantigens in rheumatoid arthritis-relevance to treatment.Autoimmun Rev. 2014 Nov;13(11):1114-20. doi: 10.1016/j.autrev.2014.08.012. Epub 2014 Aug 23. Autoimmun Rev. 2014. PMID: 25182207 Review.
-
Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis.Autoimmun Rev. 2010 Jan;9(3):140-3. doi: 10.1016/j.autrev.2009.04.006. Epub 2009 May 7. Autoimmun Rev. 2010. PMID: 19427413 Review.
Cited by
-
MixTwice: large-scale hypothesis testing for peptide arrays by variance mixing.Bioinformatics. 2021 Sep 9;37(17):2637-2643. doi: 10.1093/bioinformatics/btab162. Bioinformatics. 2021. PMID: 33693483 Free PMC article.
-
A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties.Antibodies (Basel). 2025 Aug 13;14(3):68. doi: 10.3390/antib14030068. Antibodies (Basel). 2025. PMID: 40843680 Free PMC article.
-
The peculiar features, diversity and impact of citrulline-reactive autoantibodies.Nat Rev Rheumatol. 2024 Jul;20(7):399-416. doi: 10.1038/s41584-024-01124-6. Epub 2024 Jun 10. Nat Rev Rheumatol. 2024. PMID: 38858604 Review.
-
Absolute Quantitation of Serum Antibody Reactivity Using the Richards Growth Model for Antigen Microspot Titration.Sensors (Basel). 2022 May 23;22(10):3962. doi: 10.3390/s22103962. Sensors (Basel). 2022. PMID: 35632371 Free PMC article.
-
Disordered Antigens and Epitope Overlap Between Anti-Citrullinated Protein Antibodies and Rheumatoid Factor in Rheumatoid Arthritis.Arthritis Rheumatol. 2020 Feb;72(2):262-272. doi: 10.1002/art.41074. Epub 2019 Dec 10. Arthritis Rheumatol. 2020. PMID: 31397047 Free PMC article.
References
-
- Tarcsa E., Marekov L.N., Mei G., Melino G., Lee S.C., Steinert P.M. Protein Unfolding by Peptidylarginine Deiminase. Substrate Specificity and Structural Relationships of the Natural Substrates Trichohyalin and Filaggrin. J. Biol. Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

