Role of 3-Hydroxy Fatty Acid-Induced Hepatic Lipotoxicity in Acute Fatty Liver of Pregnancy
- PMID: 29361796
- PMCID: PMC5796265
- DOI: 10.3390/ijms19010322
Role of 3-Hydroxy Fatty Acid-Induced Hepatic Lipotoxicity in Acute Fatty Liver of Pregnancy
Abstract
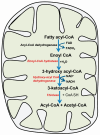
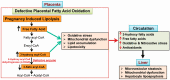
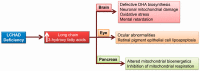
Acute fatty liver of pregnancy (AFLP), a catastrophic illness for both the mother and the unborn offspring, develops in the last trimester of pregnancy with significant maternal and perinatal mortality. AFLP is also recognized as an obstetric and medical emergency. Maternal AFLP is highly associated with a fetal homozygous mutation (1528G>C) in the gene that encodes for mitochondrial long-chain hydroxy acyl-CoA dehydrogenase (LCHAD). The mutation in LCHAD results in the accumulation of 3-hydroxy fatty acids, such as 3-hydroxy myristic acid, 3-hydroxy palmitic acid and 3-hydroxy dicarboxylic acid in the placenta, which are then shunted to the maternal circulation leading to the development of acute liver injury observed in patients with AFLP. In this review, we will discuss the mechanistic role of increased 3-hydroxy fatty acid in causing lipotoxicity to the liver and in inducing oxidative stress, mitochondrial dysfunction and hepatocyte lipoapoptosis. Further, we also review the role of 3-hydroxy fatty acids in causing placental damage, pancreatic islet β-cell glucolipotoxicity, brain damage, and retinal epithelial cells lipoapoptosis in patients with LCHAD deficiency.
Keywords: 3-hydroxy fatty acids; acute fatty liver of pregnancy; fatty acid oxidation; lipoapoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Acute fatty liver in pregnancy: revealing fetal fatty acid oxidation disorders].Arch Pediatr. 2012 Mar;19(3):277-81. doi: 10.1016/j.arcped.2011.12.020. Epub 2012 Feb 9. Arch Pediatr. 2012. PMID: 22325456 French.
-
Long-chain fatty acid oxidation during early human development.Pediatr Res. 2005 Jun;57(6):755-9. doi: 10.1203/01.PDR.0000161413.42874.74. Epub 2005 Apr 21. Pediatr Res. 2005. PMID: 15845636
-
A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women.N Engl J Med. 1999 Jun 3;340(22):1723-31. doi: 10.1056/NEJM199906033402204. N Engl J Med. 1999. PMID: 10352164
-
Mitochondrial fatty acid oxidation and acute fatty liver of pregnancy.Semin Gastrointest Dis. 2002 Jan;13(1):55-66. Semin Gastrointest Dis. 2002. PMID: 11944635 Review.
-
Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications.World J Gastroenterol. 2006 Dec 14;12(46):7397-404. doi: 10.3748/wjg.v12.i46.7397. World J Gastroenterol. 2006. PMID: 17167825 Free PMC article. Review.
Cited by
-
Prenatal Noninvasive Trio-WES in a Case of Pregnancy-Related Liver Disorder.Diagnostics (Basel). 2021 Oct 14;11(10):1904. doi: 10.3390/diagnostics11101904. Diagnostics (Basel). 2021. PMID: 34679599 Free PMC article.
-
Two Fatty Liver Conditions Masquerading as Autoimmune Hepatitis.Case Reports Hepatol. 2021 Mar 9;2021:8820350. doi: 10.1155/2021/8820350. eCollection 2021. Case Reports Hepatol. 2021. PMID: 33763269 Free PMC article.
-
Acute Fatty Liver of Pregnancy Complicated With Mild Encephalitis/Encephalopathy With a Reversible Splenial Lesion: A Case Report.Cureus. 2023 Nov 20;15(11):e49152. doi: 10.7759/cureus.49152. eCollection 2023 Nov. Cureus. 2023. PMID: 38130533 Free PMC article.
-
Risk factors in patients with acute fatty liver of pregnancy: the role of abortion, total bilirubin and serum creatinine.Arch Gynecol Obstet. 2024 Jul;310(1):153-159. doi: 10.1007/s00404-023-07234-y. Epub 2023 Nov 1. Arch Gynecol Obstet. 2024. PMID: 37910196
-
A plasma metabolomic analysis revealed the metabolic regulatory mechanism of the water extract of Dendrobium huoshanense in improving streptozotocin-induced type 1 diabetes model rats.J Nat Med. 2025 Jul;79(4):750-772. doi: 10.1007/s11418-025-01909-3. Epub 2025 May 20. J Nat Med. 2025. PMID: 40394369
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

