Testes-specific protease 50 (TSP50) promotes invasion and metastasis by inducing EMT in gastric cancer
- PMID: 29361914
- PMCID: PMC5781268
- DOI: 10.1186/s12885-018-4000-y
Testes-specific protease 50 (TSP50) promotes invasion and metastasis by inducing EMT in gastric cancer
Abstract
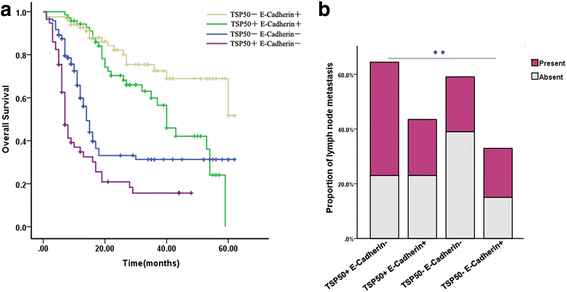
Background: TSP50 (testes-specific protease 50) has been reported to be a candidate oncogene and is overexpressed in various cancers. Our previous study demonstrated that TSP50 protein is elevated in gastric cancer, and its high expression is associated with unfavorable prognosis and lymph node metastasis. However, the role of TSP50 in gastric cancer remains elusive.
Methods: qRT-PCR, western blot were used to determine TSP50 expression in gastric cancer cell lines. Role of TSP50 in proliferation and invasion was examined by BrdU incorporation assay, cell count, wound healing and transwell assay. Immunohistochemistry and western blot were performed to identify the potential mechanisms involved.
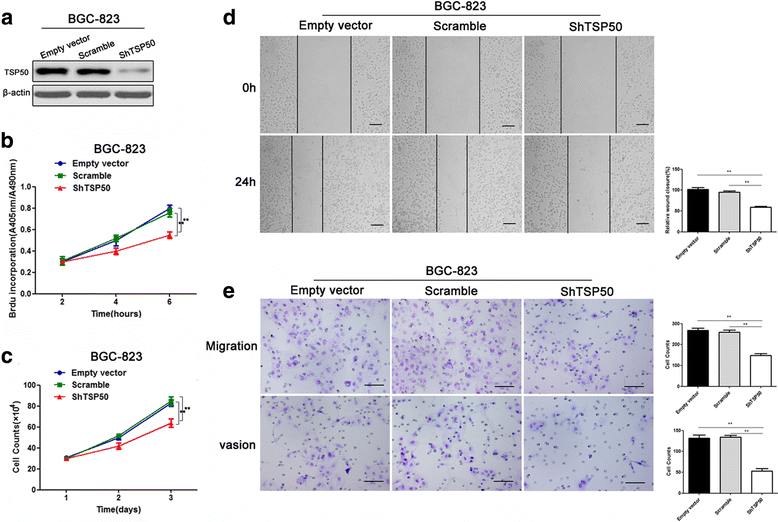
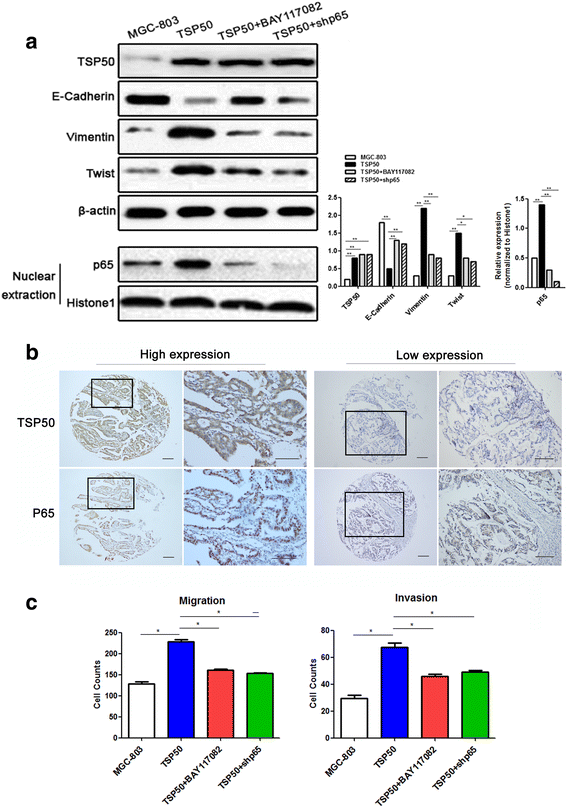
Results: TSP50 was highly expressed in most of the gastric cancer cell lines at both mRNA and protein levels. Up-regulation of TSP50 in gastric cancer cells enhanced proliferation and invasiveness, whereas down-regulation of TSP50 by its specific shRNA decreased it. A negative correlation between TSP50 and E-Cadherin was found in gastric cancer tissues, and combination of them improves the prediction for prognosis and lymph node metastasis. Mechanistic studies revealed that overexpression of TSP50 increased the expression of epithelial-to-mesenchymal transition (EMT) markers including Vimentin, and Twist, and decreased the epithelial marker E-Cadherin. NF-κB signaling pathway is involved in the regulatory effects of TSP50 on EMT, migration and invasion in gastric cancer cells.
Conclusion: TSP50 promotes the proliferation, migration and invasion of gastric cancer cells involving NF-κB dependent EMT activation. Targeting TSP50 may provide a novel therapeutic strategy for the management of gastric cancer.
Keywords: EMT; Gastric cancer; Invasion; Proliferation; TSP50.
Conflict of interest statement
Ethics approval and consent to participate
All the samples were collected with patient’s informed consent after approval from the Institute Research Medical Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University. Details of tissue microarray construction were shown in previous study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

