Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin
- PMID: 29361936
- PMCID: PMC5782380
- DOI: 10.1186/s12916-017-0999-x
Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin
Abstract
Background: Preterm prelabour rupture of the fetal membranes (PPROM) precedes 30% of preterm births and is a risk factor for early onset neonatal sepsis. As PPROM is strongly associated with ascending vaginal infection, prophylactic antibiotics are widely used. The evolution of vaginal microbiota compositions associated with PPROM and the impact of antibiotics on bacterial compositions are unknown.
Methods: We prospectively assessed vaginal microbiota prior to and following PPROM using MiSeq-based sequencing of 16S rRNA gene amplicons and examined the impact of erythromycin prophylaxis on bacterial load and community structures.
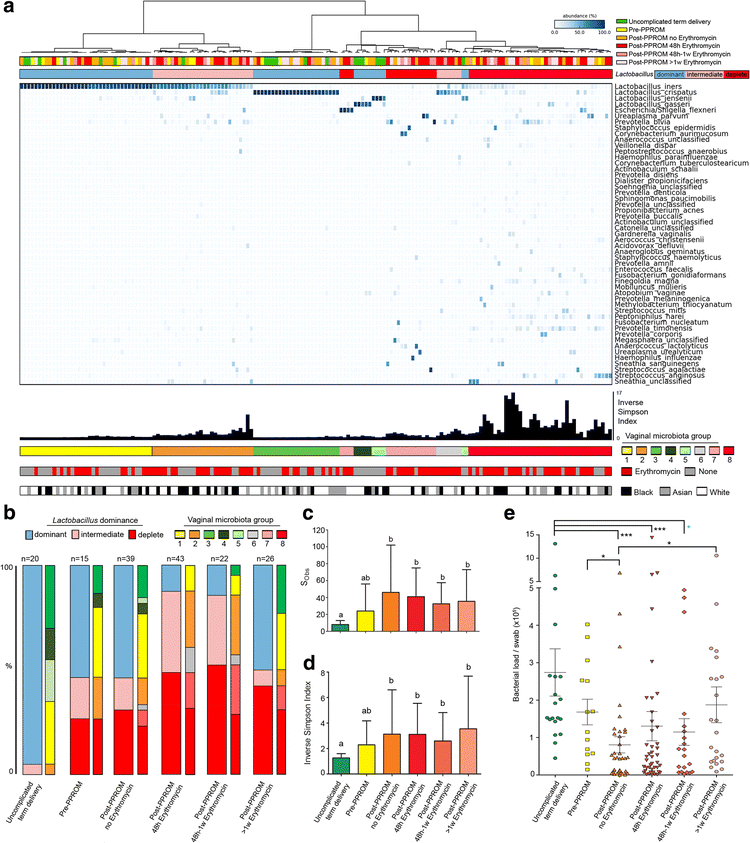
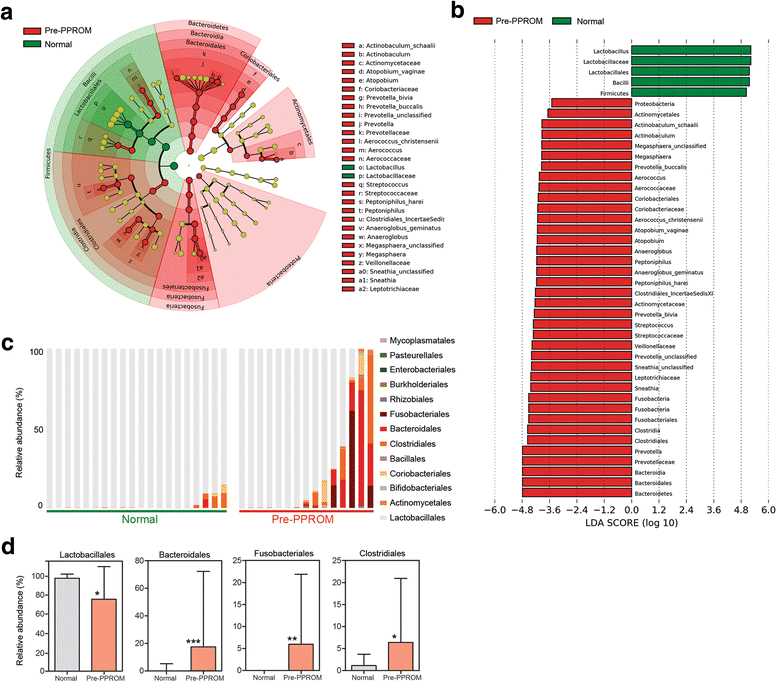
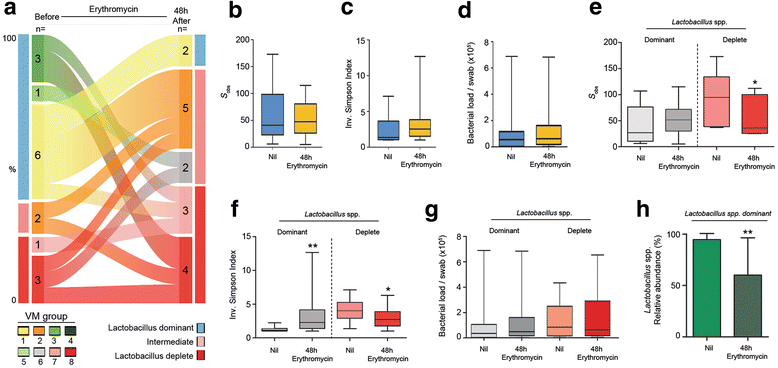
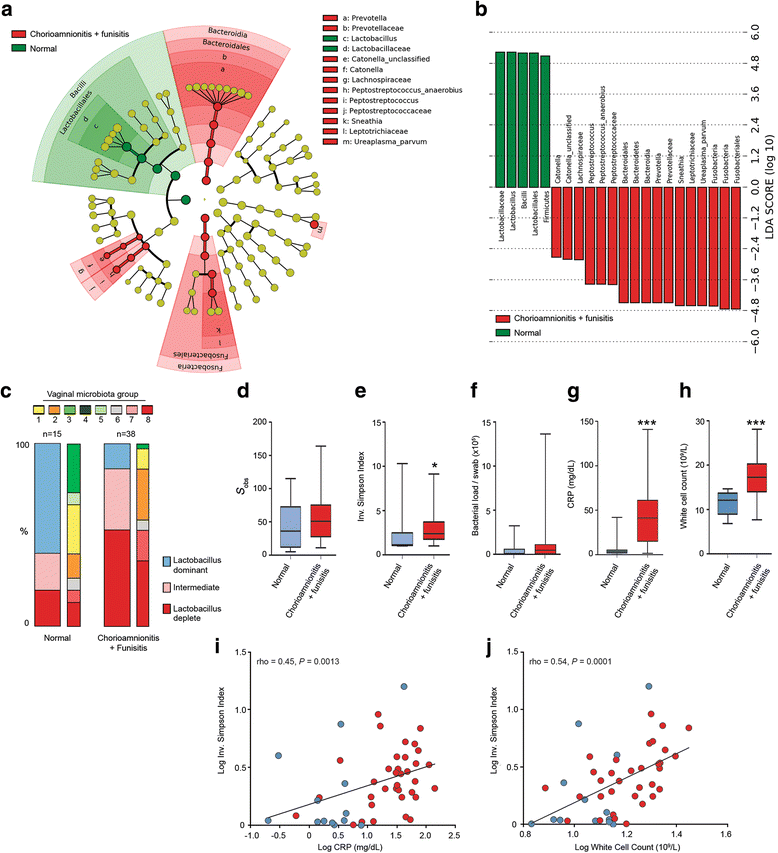
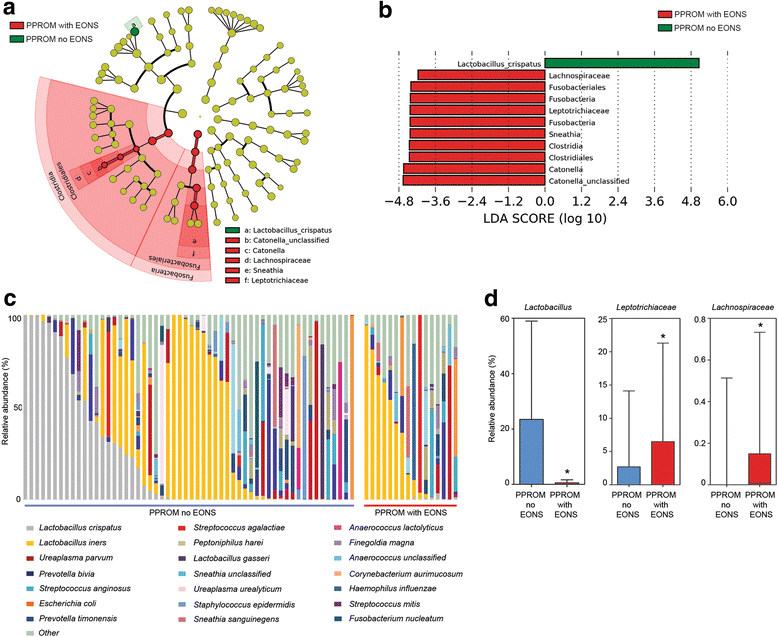
Results: In contrast to pregnancies delivering at term, vaginal dysbiosis characterised by Lactobacillus spp. depletion was present prior to the rupture of fetal membranes in approximately a third of cases (0% vs. 27%, P = 0.026) and persisted following membrane rupture (31%, P = 0.005). Vaginal dysbiosis was exacerbated by erythromycin treatment (47%, P = 0.00009) particularly in women initially colonised by Lactobacillus spp. Lactobacillus depletion and increased relative abundance of Sneathia spp. were associated with subsequent funisitis and early onset neonatal sepsis.
Conclusions: Our data show that vaginal microbiota composition is a risk factor for subsequent PPROM and is associated with adverse short-term maternal and neonatal outcomes. This highlights vaginal microbiota as a potentially modifiable antenatal risk factor for PPROM and suggests that routine use of erythromycin for PPROM be re-examined.
Keywords: Antibiotics; Erythromycin; Neonatal sepsis; Pregnancy; Preterm birth; Preterm prelabour rupture of membranes; Vaginal microbiota.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval for this studied was granted by the National Research Ethics Service Committee London–Stanmore of the National Health Service (REC 14/LO/0328), and all participants provided written informed consent.
Competing interests
PRB serves as a consultant for ObsEva, a company that works in the field of preterm birth. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Lamont RF, Duncan SLB, Mandal D, Bassett P. Intravaginal clindamycin to reduce preterm birth in women with abnormal genital tract flora. Obstet Gynecol. 2003;101(3):516–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

