Development of a complex intervention for people with chronic pain after knee replacement: the STAR care pathway
- PMID: 29361982
- PMCID: PMC5781277
- DOI: 10.1186/s13063-017-2391-8
Development of a complex intervention for people with chronic pain after knee replacement: the STAR care pathway
Abstract
Background: Approximately 20% of people who have total knee replacement experience chronic pain afterwards, but there is little evidence about effective interventions for managing this type of pain. This article describes the systematic development and refinement of a complex intervention for people with chronic pain after knee replacement. The intervention is a care pathway involving an assessment clinic and onward referral, with telephone follow-up as required. In the design of this multistage study, we chose to focus on ensuring that the intervention was deliverable, implementable and acceptable.
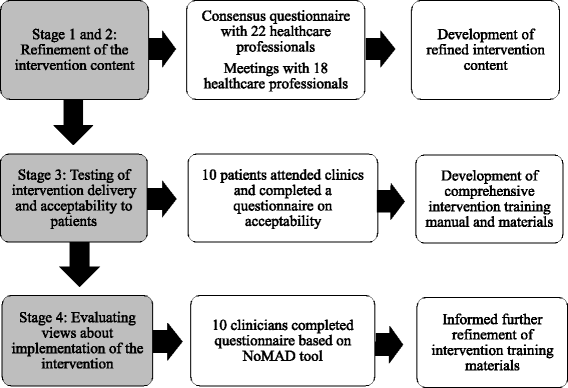
Methods: In line with the UK Medical Research Council's recommendations for comprehensive development of complex interventions, multiple phases of work were undertaken. Following on from initial development work to design the intervention, the draft intervention content was refined through consensus questionnaires with 22 health professionals and discussion at meetings with 18 healthcare professionals. Testing of intervention delivery and acceptability to patients was undertaken by two health professionals delivering the assessment clinic to ten patients. Views about future implementation within the context of a randomised trial were evaluated through a questionnaire based on the Normalisation Measure Development (NoMAD) instrument with ten health professional stakeholders.
Results: Consensus work with health professionals ensured the components of the intervention were appropriate and informed a number of substantive changes to improve the intervention. Testing of intervention delivery identified a number of logistical issues that were then addressed in the development of a comprehensive intervention training manual. Engagement with stakeholders indicated that the intervention could be successfully implemented in a clinical setting for evaluation in a randomised trial.
Conclusions: This work has informed the development and refinement of a complex intervention for people with chronic pain after knee replacement. The next stage is to evaluate the clinical and cost-effectiveness of the STAR care pathway in a multicentre randomised trial.
Keywords: Chronic pain; Complex intervention development; Total knee replacement.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was provided by the University of Bristol Faculty of Medicine and Dentistry Research Ethics Committee (stages 1 and 2; reference 21622) and NHS Research Ethics Committee (stage 3; reference 15/LO/2140). Relevant local Research and Development approvals were obtained and all participants provided informed, written consent. Stage 4 was conducted as stakeholder work with approval from the local NHS trust that this was appropriate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 13th Annual Report. Hemel Hempstead: NJR Centre; 2016.
-
- Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi: 10.1136/bmjopen-2011-000435. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical