A prion-like domain in Hsp42 drives chaperone-facilitated aggregation of misfolded proteins
- PMID: 29362223
- PMCID: PMC5881502
- DOI: 10.1083/jcb.201708116
A prion-like domain in Hsp42 drives chaperone-facilitated aggregation of misfolded proteins
Abstract
Chaperones with aggregase activity promote and organize the aggregation of misfolded proteins and their deposition at specific intracellular sites. This activity represents a novel cytoprotective strategy of protein quality control systems; however, little is known about its mechanism. In yeast, the small heat shock protein Hsp42 orchestrates the stress-induced sequestration of misfolded proteins into cytosolic aggregates (CytoQ). In this study, we show that Hsp42 harbors a prion-like domain (PrLD) and a canonical intrinsically disordered domain (IDD) that act coordinately to promote and control protein aggregation. Hsp42 PrLD is essential for CytoQ formation and is bifunctional, mediating self-association as well as binding to misfolded proteins. Hsp42 IDD confines chaperone and aggregase activity and affects CytoQ numbers and stability in vivo. Hsp42 PrLD and IDD are both crucial for cellular fitness during heat stress, demonstrating the need for sequestering misfolded proteins in a regulated manner.
© 2018 Grousl et al.
Figures
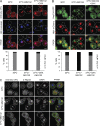
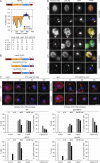
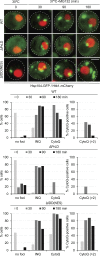

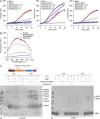
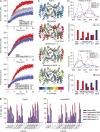
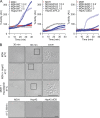
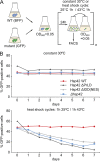
Comment in
-
One domain fits all: Using disordered regions to sequester misfolded proteins.J Cell Biol. 2018 Apr 2;217(4):1173-1175. doi: 10.1083/jcb.201803015. Epub 2018 Mar 9. J Cell Biol. 2018. PMID: 29523584 Free PMC article.
Similar articles
-
Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae.J Cell Biol. 2011 Nov 14;195(4):617-29. doi: 10.1083/jcb.201106037. Epub 2011 Nov 7. J Cell Biol. 2011. PMID: 22065637 Free PMC article.
-
Spatially organized aggregation of misfolded proteins as cellular stress defense strategy.J Mol Biol. 2015 Apr 10;427(7):1564-74. doi: 10.1016/j.jmb.2015.02.006. Epub 2015 Feb 11. J Mol Biol. 2015. PMID: 25681695 Review.
-
Hsp42 is the general small heat shock protein in the cytosol of Saccharomyces cerevisiae.EMBO J. 2004 Feb 11;23(3):638-49. doi: 10.1038/sj.emboj.7600080. Epub 2004 Jan 29. EMBO J. 2004. PMID: 14749732 Free PMC article.
-
Compartment-specific aggregases direct distinct nuclear and cytoplasmic aggregate deposition.EMBO J. 2015 Mar 12;34(6):778-97. doi: 10.15252/embj.201489524. Epub 2015 Feb 11. EMBO J. 2015. PMID: 25672362 Free PMC article.
-
Role of sHsps in organizing cytosolic protein aggregation and disaggregation.Cell Stress Chaperones. 2017 Jul;22(4):493-502. doi: 10.1007/s12192-017-0762-4. Epub 2017 Jan 24. Cell Stress Chaperones. 2017. PMID: 28120291 Free PMC article. Review.
Cited by
-
Regulation of physiological and pathological condensates by molecular chaperones.FEBS J. 2025 Jul;292(13):3271-3297. doi: 10.1111/febs.17390. Epub 2025 Jan 5. FEBS J. 2025. PMID: 39756021 Free PMC article. Review.
-
Neuromuscular Diseases Due to Chaperone Mutations: A Review and Some New Results.Int J Mol Sci. 2020 Feb 19;21(4):1409. doi: 10.3390/ijms21041409. Int J Mol Sci. 2020. PMID: 32093037 Free PMC article. Review.
-
Cytoplasmic redox imbalance in the thioredoxin system activates Hsf1 and results in hyperaccumulation of the sequestrase Hsp42 with misfolded proteins.Mol Biol Cell. 2024 Apr 1;35(4):ar53. doi: 10.1091/mbc.E23-07-0296. Epub 2024 Feb 21. Mol Biol Cell. 2024. PMID: 38381577 Free PMC article.
-
Cellular sequestrases maintain basal Hsp70 capacity ensuring balanced proteostasis.Nat Commun. 2019 Oct 24;10(1):4851. doi: 10.1038/s41467-019-12868-1. Nat Commun. 2019. PMID: 31649258 Free PMC article.
-
N- and C-terminal regions of the small heat shock protein IbpA from Acholeplasma laidlawii competitively govern its oligomerization pattern and chaperone-like activity.RSC Adv. 2020 Feb 26;10(14):8364-8376. doi: 10.1039/c9ra10172a. eCollection 2020 Feb 24. RSC Adv. 2020. PMID: 35497866 Free PMC article.
References
-
- Cherkasov V., Grousl T., Theer P., Vainshtein Y., Glässer C., Mongis C., Kramer G., Stoecklin G., Knop M., Mogk A., and Bukau B.. 2015. Systemic control of protein synthesis through sequestration of translation and ribosome biogenesis factors during severe heat stress. FEBS Lett. 589:3654–3664. 10.1016/j.febslet.2015.10.010 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

