ELOVL4-Mediated Production of Very Long-Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability
- PMID: 29362226
- PMCID: PMC5860862
- DOI: 10.2337/db17-1034
ELOVL4-Mediated Production of Very Long-Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability
Abstract
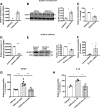
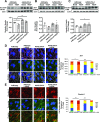
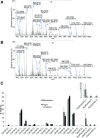
Tight junctions (TJs) involve close apposition of transmembrane proteins between cells. Although TJ proteins have been studied in detail, the role of lipids is largely unknown. We addressed the role of very long-chain (VLC ≥26) ceramides in TJs using diabetes-induced loss of the blood-retinal barrier as a model. VLC fatty acids that incorporate into VLC ceramides are produced by elongase elongation of very long-chain fatty acids protein 4 (ELOVL4). ELOVL4 is significantly reduced in the diabetic retina. Overexpression of ELOVL4 significantly decreased basal permeability, inhibited vascular endothelial growth factor (VEGF)- and interleukin-1β-induced permeability, and prevented VEGF-induced decrease in occludin expression and border staining of TJ proteins ZO-1 and claudin-5. Intravitreal delivery of AAV2-hELOVL4 reduced diabetes-induced increase in vascular permeability. Ultrastructure and lipidomic analysis revealed that ω-linked acyl-VLC ceramides colocalize with TJ complexes. Overall, normalization of retinal ELOVL4 expression could prevent blood-retinal barrier dysregulation in diabetic retinopathy through an increase in VLC ceramides and stabilization of TJs.
© 2018 by the American Diabetes Association.
Figures



References
-
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999;274:23463–23467 - PubMed
-
- Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci 2006;47:5106–5115 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

