Processing of Candida albicans Ece1p Is Critical for Candidalysin Maturation and Fungal Virulence
- PMID: 29362237
- PMCID: PMC5784256
- DOI: 10.1128/mBio.02178-17
Processing of Candida albicans Ece1p Is Critical for Candidalysin Maturation and Fungal Virulence
Abstract
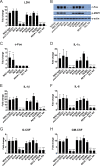
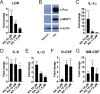
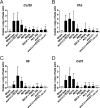
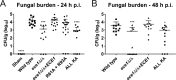
Candida albicans is an opportunistic fungal pathogen responsible for superficial and life-threatening infections in humans. During mucosal infection, C. albicans undergoes a morphological transition from yeast to invasive filamentous hyphae that secrete candidalysin, a 31-amino-acid peptide toxin required for virulence. Candidalysin damages epithelial cell plasma membranes and stimulates the activating protein 1 (AP-1) transcription factor c-Fos (via p38-mitogen-activated protein kinase [MAPK]), and the MAPK phosphatase MKP1 (via extracellular signal-regulated kinases 1 and 2 [ERK1/2]-MAPK), which trigger and regulate proinflammatory cytokine responses, respectively. The candidalysin toxin resides as a discrete cryptic sequence within a larger 271-amino-acid parental preproprotein, Ece1p. Here, we demonstrate that kexin-like proteinases, but not secreted aspartyl proteinases, initiate a two-step posttranslational processing of Ece1p to produce candidalysin. Kex2p-mediated proteolysis of Ece1p after Arg61 and Arg93, but not after other processing sites within Ece1p, is required to generate immature candidalysin from Ece1p, followed by Kex1p-mediated removal of a carboxyl arginine residue to generate mature candidalysin. C. albicans strains harboring mutations of Arg61 and/or Arg93 did not secrete candidalysin, were unable to induce epithelial damage and inflammatory responses in vitro, and showed attenuated virulence in vivo in a murine model of oropharyngeal candidiasis. These observations identify enzymatic processing of C. albicans Ece1p by kexin-like proteinases as crucial steps required for candidalysin production and fungal pathogenicity.IMPORTANCECandida albicans is an opportunistic fungal pathogen that causes mucosal infection in millions of individuals worldwide. Successful infection requires the secretion of candidalysin, the first cytolytic peptide toxin identified in any human fungal pathogen. Candidalysin is derived from its parent protein Ece1p. Here, we identify two key amino acids within Ece1p vital for processing and production of candidalysin. Mutations of these residues render C. albicans incapable of causing epithelial damage and markedly reduce mucosal infection in vivo Importantly, candidalysin production requires two individual enzymatic events. The first involves processing of Ece1p by Kex2p, yielding immature candidalysin, which is then further processed by Kex1p to produce the mature toxin. These observations identify important steps for C. albicans pathogenicity at mucosal surfaces.
Keywords: Candida albicans; candidalysin; fungal infection; kexin; mucosal immunity.
Copyright © 2018 Richardson et al.
Figures
References
-
- Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Förster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Häder A, Kurzai O, Luo T, Krüger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. 2016. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532:64–68. doi:10.1038/nature17625. - DOI - PMC - PubMed
-
- Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. 2010. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 8:225–235. doi:10.1016/j.chom.2010.08.002. - DOI - PMC - PubMed
-
- Sanders SL, Schekman R. 1992. Polypeptide translocation across the endoplasmic reticulum membrane. J Biol Chem 267:13791–13794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 102549/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- R01 DE022550/DE/NIDCR NIH HHS/United States
- R37 DE022550/DE/NIDCR NIH HHS/United States
- MR/J008303/1/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- BB/J016411/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 DE023815/DE/NIDCR NIH HHS/United States
- MR/N006364/1/MRC_/Medical Research Council/United Kingdom
- MR/M011372/1/MRC_/Medical Research Council/United Kingdom
- BB-J016411-1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097377/Z/11/Z/WT_/Wellcome Trust/United Kingdom
- BB/N014677/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
