Adaptive Evolution of RH5 in Ape Plasmodium species of the Laverania Subgenus
- PMID: 29362238
- PMCID: PMC5784257
- DOI: 10.1128/mBio.02237-17
Adaptive Evolution of RH5 in Ape Plasmodium species of the Laverania Subgenus
Abstract
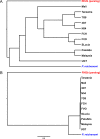
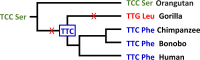
Plasmodium falciparum, the major cause of malaria morbidity and mortality in humans, has been shown to have emerged after cross-species transmission of one of six host-specific parasites (subgenus Laverania) infecting wild chimpanzees (Pan troglodytes) and western gorillas (Gorilla gorilla). Binding of the parasite-encoded ligand RH5 to the host protein basigin is essential for erythrocyte invasion and has been implicated in host specificity. A recent study claimed to have found two amino acid changes in RH5 that "drove the host shift leading to the emergence of P. falciparum as a human pathogen." However, the ape Laverania data available at that time, which included only a single distantly related chimpanzee parasite sequence, were inadequate to justify any such conclusion. Here, we have investigated Laverania Rh5 gene evolution using sequences from all six ape parasite species. Searching for gene-wide episodic selection across the entire Laverania phylogeny, we found eight codons to be under positive selection, including three that correspond to contact residues known to form hydrogen bonds between P. falciparum RH5 and human basigin. One of these sites (residue 197) has changed subsequent to the transmission from apes to humans that gave rise to P. falciparum, suggesting a possible role in the adaptation of the gorilla parasite to the human host. We also found evidence that the patterns of nucleotide polymorphisms in P. falciparum are not typical of Laverania species and likely reflect the recent demographic history of the human parasite.IMPORTANCE A number of closely related, host-specific malaria parasites infecting wild chimpanzees and gorillas have recently been described. The most important cause of human malaria, Plasmodium falciparum, is now known to have resulted from a cross-species transmission of one of the gorilla parasites. Overcoming species-specific interactions between a parasite ligand, RH5, and its receptor on host cells, basigin, was likely an important step in the origin of the human parasite. We have investigated the evolution of the Rh5 gene and found evidence of adaptive changes during the diversification of the ape parasite species at sites that are known to form bonds with human basigin. One of these changes occurred at the origin of P. falciparum, implicating it as an important adaptation to the human host.
Keywords: Laverania; Plasmodium falciparum; RH5; basigin; chimpanzee; gorilla.
Copyright © 2018 Plenderleith et al.
Figures



Comment in
-
Reply to Forni et al., "Multiple Selected Changes May Modulate the Molecular Interaction between Laverania RH5 and Primate Basigin".mBio. 2018 May 22;9(3):e00917-18. doi: 10.1128/mBio.00917-18. mBio. 2018. PMID: 29789362 Free PMC article. No abstract available.
-
Multiple Selected Changes May Modulate the Molecular Interaction between Laverania RH5 and Primate Basigin.mBio. 2018 May 22;9(3):e00476-18. doi: 10.1128/mBio.00476-18. mBio. 2018. PMID: 29789367 Free PMC article. No abstract available.
Similar articles
-
Positive selection underlies the species-specific binding of Plasmodium falciparum RH5 to human basigin.Mol Ecol. 2015 Sep;24(18):4711-22. doi: 10.1111/mec.13354. Epub 2015 Sep 7. Mol Ecol. 2015. PMID: 26302433
-
RH5-Basigin interaction plays a major role in the host tropism of Plasmodium falciparum.Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20735-40. doi: 10.1073/pnas.1320771110. Epub 2013 Dec 2. Proc Natl Acad Sci U S A. 2013. PMID: 24297912 Free PMC article.
-
Origin of the human malaria parasite Plasmodium falciparum in gorillas.Nature. 2010 Sep 23;467(7314):420-5. doi: 10.1038/nature09442. Nature. 2010. PMID: 20864995 Free PMC article.
-
Ape Origins of Human Malaria.Annu Rev Microbiol. 2020 Sep 8;74:39-63. doi: 10.1146/annurev-micro-020518-115628. Annu Rev Microbiol. 2020. PMID: 32905751 Free PMC article. Review.
-
Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax.Int J Parasitol. 2017 Feb;47(2-3):87-97. doi: 10.1016/j.ijpara.2016.05.008. Epub 2016 Jul 2. Int J Parasitol. 2017. PMID: 27381764 Free PMC article. Review.
Cited by
-
Ancient Introgression between Two Ape Malaria Parasite Species.Genome Biol Evol. 2019 Nov 1;11(11):3269-3274. doi: 10.1093/gbe/evz244. Genome Biol Evol. 2019. PMID: 31697367 Free PMC article.
-
The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target.Trends Parasitol. 2020 Jun;36(6):545-559. doi: 10.1016/j.pt.2020.04.003. Epub 2020 Apr 28. Trends Parasitol. 2020. PMID: 32359873 Free PMC article. Review.
-
Reply to Forni et al., "Multiple Selected Changes May Modulate the Molecular Interaction between Laverania RH5 and Primate Basigin".mBio. 2018 May 22;9(3):e00917-18. doi: 10.1128/mBio.00917-18. mBio. 2018. PMID: 29789362 Free PMC article. No abstract available.
-
The Genome of Plasmodium gonderi: Insights into the Evolution of Human Malaria Parasites.Genome Biol Evol. 2024 Feb 1;16(2):evae027. doi: 10.1093/gbe/evae027. Genome Biol Evol. 2024. PMID: 38376987 Free PMC article.
-
Plasmodium-a brief introduction to the parasites causing human malaria and their basic biology.J Physiol Anthropol. 2021 Jan 7;40(1):1. doi: 10.1186/s40101-020-00251-9. J Physiol Anthropol. 2021. PMID: 33413683 Free PMC article. Review.
References
-
- Coatney GR, Collins WE, Warren M, Contacos PG. 1971. The primate malarias. US Government Printing Office, Washington, DC: http://phsource.us/PH/PARA/Book/primate_24.pdf.
-
- Duval L, Fourment M, Nerrienet E, Rousset D, Sadeuh SA, Goodman SM, Andriaholinirina NV, Randrianarivelojosia M, Paul RE, Robert V, Ayala FJ, Ariey F. 2010. African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc Natl Acad Sci U S A 107:10561–10566. doi:10.1073/pnas.1005435107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
