Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA
- PMID: 29362248
- PMCID: PMC5786138
- DOI: 10.1136/bmjopen-2017-017653
Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare AGENDA
Abstract
Objectives: To determine if patient-centred communication leads to a reduction of the number of medications taken without reducing health-related quality of life.
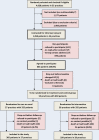
Design: Two-arm cluster-randomised controlled trial.
Setting: 55 primary care practices in Hamburg, Düsseldorf and Rostock, Germany.
Participants: 604 patients 65 to 84 years of age with at least three chronic conditions.
Interventions: Within the 12-month intervention, general practitioners (GPs) had three 30 min talks with each of their patients in addition to routine consultations. The first talk aimed at identifying treatment targets and priorities of the patient. During the second talk, the medication taken by the patient was discussed based on a 'brown bag' review of all the medications the patient had at home. The third talk served to discuss goal attainment and future treatment targets. GPs in the control group performed care as usual.
Primary outcome measures: We assumed that the number of medications taken by the patient would be reduced by 1.5 substances in the intervention group and that the change in the intervention group's health-related quality of life would not be statistically significantly inferior to the control group.
Results: The patients took a mean of 7.0±3.5 medications at baseline and 6.8±3.5 medications at follow-up. There was no difference between treatment and control group in the change of the number of medications taken (0.43; 95% CI -0.07 to 0.93; P=0.094) and no difference in health-related quality of life (0.03; -0.02 to 0.08; P=0.207). The likelihood of receiving a new prescription for analgesics was twice as high in the intervention group compared with the control group (risk ratio, 2.043; P=0.019), but the days spent in hospital were reduced by the intervention (-3.07; -5.25 to -0.89; P=0.006).
Conclusions: Intensifying the doctor-patient dialogue and discussing the patient's agenda and personal needs did not lead to a reduction of medication intake and did not alter health-related quality of life.
Trial registration number: ISRCTN46272088; Pre-results.
Keywords: chronic care model; multimorbidity; narrative based medicine; polypharmacy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
