Cost-effectiveness of FreeO2 in patients with chronic obstructive pulmonary disease hospitalised for acute exacerbations: analysis of a pilot study in Quebec
- PMID: 29362258
- PMCID: PMC5786115
- DOI: 10.1136/bmjopen-2017-018835
Cost-effectiveness of FreeO2 in patients with chronic obstructive pulmonary disease hospitalised for acute exacerbations: analysis of a pilot study in Quebec
Abstract
Objective: Conduct a cost-effectiveness analysis of FreeO2 technology versus manual oxygen-titration technology for patients with chronic obstructive pulmonary disease (COPD) hospitalised for acute exacerbations.
Setting: Tertiary acute care hospital in Quebec, Canada.
Participants: 47 patients with COPD hospitalised for acute exacerbations.
Intervention: An automated oxygen-titration and oxygen-weaning technology.
Methods and outcomes: The costs for hospitalisation and follow-up for 180 days were calculated using a microcosting approach and included the cost of FreeO2 technology. Incremental cost-effectiveness ratios (ICERs) were calculated using bootstrap resampling with 5000 replications. The main effect variable was the percentage of time spent at the target oxygen saturation (SpO2). The other two effect variables were the time spent in hyperoxia (target SpO2+5%) and in severe hypoxaemia (SpO2 <85%). The resamplings were based on data from a randomised controlled trial with 47 patients with COPD hospitalised for acute exacerbations.
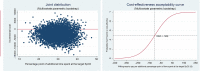
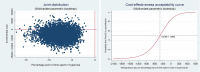
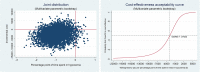
Results: FreeO2 generated savings of 20.7% of the per-patient costs at 180 days (ie, -$C2959.71). This decrease is nevertheless not significant at the 95% threshold (P=0.13), but the effect variables all improved (P<0.001). The improvement in the time spent at the target SpO2 was 56.3%. The ICERs indicate that FreeO2 technology is more cost-effective than manual oxygen titration with a savings of -$C96.91 per percentage point of time spent at the target SpO2 (95% CI -301.26 to 116.96).
Conclusion: FreeO2 technology could significantly enhance the efficiency of the health system by reducing per-patient costs at 180 days. A study with a larger patient sample needs to be carried out to confirm these preliminary results.
Trial registration number: NCT01393015; Post-results.
Keywords: bootstrap; canada; copd; cost-effectiveness; freeo2; oxygen-titration.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: FL is the coinventor of the FreeO2 system and cofounder of Oxynov, the firm that developed and productionised the FreeO2 system for marketing. The Fonds de Recherche en Santé du Québec contributes to Dr François Lellouche salary for research activities (clinical research scholar) and to the research assistant’s salary (clinical research grant). FM holds a GlaxoSmithKline/Canadian Institutes of Health Research chair on COPD at Laval University. FM participates in Innovair, a company that owns shares in OxyNov, the owner of the FreeO2 device.
Figures
Similar articles
-
Automated oxygen titration and weaning with FreeO2 in patients with acute exacerbation of COPD: a pilot randomized trial.Int J Chron Obstruct Pulmon Dis. 2016 Aug 24;11:1983-90. doi: 10.2147/COPD.S112820. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27601891 Free PMC article. Clinical Trial.
-
Automated oxygen administration versus conventional oxygen therapy after major abdominal or thoracic surgery: study protocol for an international multicentre randomised controlled study.BMJ Open. 2019 Jan 17;9(1):e023833. doi: 10.1136/bmjopen-2018-023833. BMJ Open. 2019. PMID: 30782716 Free PMC article.
-
Automatic Oxygen Titration During Walking in Subjects With COPD: A Randomized Crossover Controlled Study.Respir Care. 2016 Nov;61(11):1456-1464. doi: 10.4187/respcare.04406. Epub 2016 Oct 18. Respir Care. 2016. PMID: 27794080 Clinical Trial.
-
The risk of serious adverse outcomes associated with hypoxaemia and hyperoxaemia in acute exacerbations of COPD.Postgrad Med J. 2012 Dec;88(1046):684-9. doi: 10.1136/postgradmedj-2012-130809. Epub 2012 Sep 12. Postgrad Med J. 2012. PMID: 22977283 Review.
-
Cost-effectiveness of long-term oxygen therapy for chronic obstructive disease.Am J Manag Care. 2009 Feb;15(2):97-104. Am J Manag Care. 2009. PMID: 19284806 Review.
Cited by
-
Eosinophil counts in first COPD hospitalizations: a 1-year cost analysis in Quebec, Canada.Int J Chron Obstruct Pulmon Dis. 2018 Oct 8;13:3065-3076. doi: 10.2147/COPD.S170747. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 30349220 Free PMC article.
-
Clinical Implementation of Automated Oxygen Titration in a Tertiary Care Hospital.Respir Care. 2024 Aug 24;69(9):1081-1091. doi: 10.4187/respcare.11331. Respir Care. 2024. PMID: 38490735
-
Economic Burden of Chronic Obstructive Pulmonary Disease: A Systematic Review.Tuberc Respir Dis (Seoul). 2024 Jul;87(3):234-251. doi: 10.4046/trd.2023.0100. Epub 2024 Feb 16. Tuberc Respir Dis (Seoul). 2024. PMID: 38361331 Free PMC article.
-
Closed-loop oxygen control improves oxygenation in pediatric patients under high-flow nasal oxygen-A randomized crossover study.Front Med (Lausanne). 2022 Nov 16;9:1046902. doi: 10.3389/fmed.2022.1046902. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36465920 Free PMC article.
-
Closed-loop oxygen usage during invasive mechanical ventilation of pediatric patients (CLOUDIMPP): a randomized controlled cross-over study.Front Med (Lausanne). 2024 Sep 10;11:1426969. doi: 10.3389/fmed.2024.1426969. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39318593 Free PMC article.
References
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD). 2016. http://www.who.int/respiratory/copd/en/ (accessed 23 Nov 2016).
-
- Statistique Canada. Enquête sur la santé dans les collectivités canadiennes. 2010. http://www.cadretravailpulmonaire.ca/santerespiratoireauCanada/ (accessed 23 Nov 2016).
-
- Agence de la santé publique du Canada. Faits saillants sur la maladie pulmonaire obstructive chronique (MPOC). 2011. http://www.phac-aspc.gc.ca/cd-mc/publications/copd-mpoc/ff-rr-2011-fra.php (accessed 23 Nov 2016).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
