Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering
- PMID: 29362293
- PMCID: PMC5884090
- DOI: 10.1088/1748-605X/aaaa29
Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering
Abstract
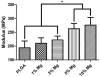
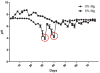
Controlling acidic degradation of biodegradable polyesters remains a major clinical challenge. This work presents a simple and effective strategy of developing polyester composites with biodegradable magnesium metal or alloys. PLGA samples with compositions of 1, 3, 5, and 10 wt% magnesium were produced using a simple solvent-casting method, which resulted in composite films with near uniform Mg metal/alloy particle dispersion. Degradation study of the composite films showed that all compositions higher than 1 wt% magnesium were able to extend the duration of degradation, and buffer acidic pH resulting from PLGA degradation. PLGA composite with 5 wt% of magnesium showed near-neutral degradation pattern under sink conditions. Magnesium addition also showed improved mechanical characteristics in terms of the tensile modulus. In vitro experiments conducted by seeding PLGA composites with MC3T3-E1 pre-osteoblasts demonstrated increased ALP expression and cellular mineralization. The established new biodegradable polymer-metal system provides a useful biomaterial platform with a wide range of applications in biomedical device development and scaffold-based tissue engineering.
Figures

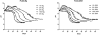

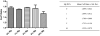



References
-
- Kumbar SG, Laurencin CT, Deng M. Natural and Synthetic Biomedical Polymers. Oxford: Elsevier; 2014.
-
- Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 5:1–16. discussion 16, May 2003. - PubMed
-
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000 Dec;21(24):2529–2543. - PubMed
-
- Nukavarapu SP, Freeman J, Laurencin CT. Regenerative Engineering of Musculoskeletal Tissues and Interfaces. Elsevier Science & Technology; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
