Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases
- PMID: 29362397
- PMCID: PMC5833873
- DOI: 10.1038/s41389-017-0025-3
Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases
Abstract
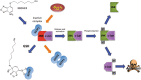
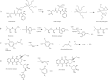
Glutathione transferase classical GSH conjugation activity plays a critical role in cellular detoxification against xenobiotics and noxious compounds as well as against oxidative stress. However, this feature is also exploited by cancer cells to acquire drug resistance and improve their survival. As a result, various members of the family were found overexpressed in a number of different cancers. Moreover several GST polymorphisms, ranging from null phenotypes to point mutations, were detected in members of the family and found to correlate with the onset of neuro-degenerative diseases. In the last decades, a great deal of research aimed at clarifying the role played by GSTs in drug resistance, at developing inhibitors to counteract this activity but also at exploiting GSTs for prodrugs specific activation in cancer cells. Here we summarize some of the most important achievements reached in this lively area of research.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
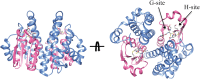



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

