Sequence-regulated copolymerization based on periodic covalent positioning of monomers along one-dimensional nanochannels
- PMID: 29362404
- PMCID: PMC5780473
- DOI: 10.1038/s41467-017-02736-1
Sequence-regulated copolymerization based on periodic covalent positioning of monomers along one-dimensional nanochannels
Abstract
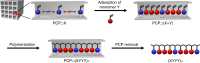
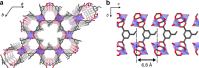
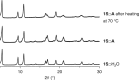
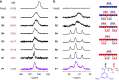
The design of monomer sequences in polymers has been a challenging research subject, especially in making vinyl copolymers by free-radical polymerization. Here, we report a strategy to obtain sequence-regulated vinyl copolymers, utilizing the periodic structure of a porous coordination polymer (PCP) as a template. Mixing of Cu2+ ion and styrene-3,5-dicarboxylic acid (S) produces a PCP, [Cu(styrene-3,5-dicarboxylate)] n , with the styryl groups periodically immobilized along the one-dimensional channels. After the introduction of acrylonitrile (A) into the host PCP, radical copolymerization between A and the immobilized S is performed inside the channel, followed by decomposing the PCP to isolate the resulting copolymer. The predominant repetitive SAAA sequence in the copolymer is confirmed by monomer composition, NMR spectroscopy and theoretical calculations. Copolymerization using methyl vinyl ketone also provides the same type of sequence-regulated copolymer, showing that this methodology has a versatility to control the copolymer sequence via transcription of PCP periodicity at the molecular level.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Synthesis and Functionalization of Periodic Copolymers.Polymers (Basel). 2017 May 6;9(5):166. doi: 10.3390/polym9050166. Polymers (Basel). 2017. PMID: 30970845 Free PMC article.
-
Sequence-regulated vinyl copolymers by metal-catalysed step-growth radical polymerization.Nat Commun. 2010 Apr 12;1:6. doi: 10.1038/ncomms1004. Nat Commun. 2010. PMID: 20975670
-
Monomer Sequence Regulation in Main and Side Chains of Vinyl Copolymers: Synthesis of Vinyl Oligomonomers via Sequential Atom Transfer Radical Addition and Their Alternating Radical Copolymerization.ACS Macro Lett. 2015 Jul 21;4(7):745-749. doi: 10.1021/acsmacrolett.5b00379. Epub 2015 Jul 1. ACS Macro Lett. 2015. PMID: 35596500
-
Oxygen-Triggered Switchable Polymerization for the One-Pot Synthesis of CO2 -Based Block Copolymers from Monomer Mixtures.Angew Chem Int Ed Engl. 2019 Oct 1;58(40):14311-14318. doi: 10.1002/anie.201906140. Epub 2019 Aug 21. Angew Chem Int Ed Engl. 2019. PMID: 31282122 Review.
-
Copolymerization of ethylene with non-vinyl polar monomers.Proc Jpn Acad Ser B Phys Biol Sci. 2022;98(5):222-226. doi: 10.2183/pjab.98.014. Proc Jpn Acad Ser B Phys Biol Sci. 2022. PMID: 35545528 Free PMC article. Review.
Cited by
-
Sequence-Controlled Copolymerization of Structurally Well-Defined Multinuclear Zinc Acrylate Complexes and Styrene.Macromol Rapid Commun. 2025 Feb;46(3):e2400742. doi: 10.1002/marc.202400742. Epub 2024 Nov 9. Macromol Rapid Commun. 2025. PMID: 39520319 Free PMC article.
-
Polymerization within Nanoporous Anodized Alumina Oxide Templates (AAO): A Critical Survey.Polymers (Basel). 2023 Jan 19;15(3):525. doi: 10.3390/polym15030525. Polymers (Basel). 2023. PMID: 36771824 Free PMC article. Review.
-
Transparent to Black Electrochromism-The "Holy Grail" of Organic Optoelectronics.Polymers (Basel). 2019 Feb 6;11(2):273. doi: 10.3390/polym11020273. Polymers (Basel). 2019. PMID: 30960257 Free PMC article. Review.
-
The Different Outcomes of Electrochemical Copolymerisation: 3-Hexylthiophene with Indole, Carbazole or Fluorene.Polymers (Basel). 2019 Feb 18;11(2):355. doi: 10.3390/polym11020355. Polymers (Basel). 2019. PMID: 30960339 Free PMC article.
-
Single Atom Catalysts Based on Earth-Abundant Metals for Energy-Related Applications.Chem Rev. 2024 Nov 13;124(21):11767-11847. doi: 10.1021/acs.chemrev.4c00155. Epub 2024 Jul 5. Chem Rev. 2024. PMID: 38967551 Free PMC article. Review.
References
-
- Alberts B, et al. Molecular Biology of the Cell. 4th edn. New York: Garland Science; 2002.
-
- Merrifield RB. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963;85:2149–2154. doi: 10.1021/ja00897a025. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

