Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses
- PMID: 29362446
- PMCID: PMC5837162
- DOI: 10.1038/s41426-017-0007-8
Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses
Abstract
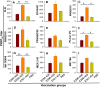
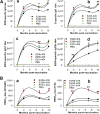
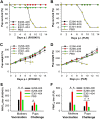
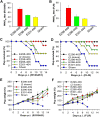
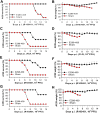
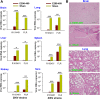
Zika virus (ZIKV) infection remains a serious health threat due to its close association with congenital Zika syndrome (CZS), which includes microcephaly and other severe birth defects. As no vaccines are available for human use, continuous effort is needed to develop effective and safe vaccines to prevent ZIKV infection. In this study, we constructed three recombinant proteins comprising, respectively, residues 296-406 (E296-406), 298-409 (E298-409), and 301-404 (E301-404) of ZIKV envelope (E) protein domain III (EDIII) fused with a C-terminal Fc of human IgG. Our results demonstrated that E298-409 induced the highest titer of neutralizing antibodies against infection with nine ZIKV strains isolated from different hosts, countries, and time periods, and it maintained long-term anti-ZIKV immunogenicity to induce neutralizing antibodies. Pups born to mice immunized with E298-409 were fully protected against lethal challenge with two epidemic human ZIKV strains, 2015/Honduras (R103451) and 2015/Colombia (FLR). Passive transfer of anti-E298-409 mouse sera protected pups born to naive mice, as well as type I interferon receptor-deficient adult A129 mice, from lethal challenge with human ZIKV strains R103451 and FLR, and this protection was positively correlated with neutralizing antibodies. These data suggest that the critical neutralizing fragment (i.e., a fragment that can induce highly potent neutralizing antibodies against divergent ZIKV strains) of ZIKV EDIII is a good candidate for development as an effective and safe ZIKV subunit vaccine to protect pregnant mothers and their fetuses against ZIKV infection. The E298-409-specific antibodies can be used for passive immunization to prevent ZIKV infection in newborns or immunocompromised adults.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Rational Design of Zika Virus Subunit Vaccine with Enhanced Efficacy.J Virol. 2019 Aug 13;93(17):e02187-18. doi: 10.1128/JVI.02187-18. Print 2019 Sep 1. J Virol. 2019. PMID: 31189716 Free PMC article.
-
Insect cell-produced recombinant protein subunit vaccines protect against Zika virus infection.Antiviral Res. 2018 Jun;154:97-103. doi: 10.1016/j.antiviral.2018.04.010. Epub 2018 Apr 14. Antiviral Res. 2018. PMID: 29665376
-
Yeast-produced subunit protein vaccine elicits broadly neutralizing antibodies that protect mice against Zika virus lethal infection.Antiviral Res. 2019 Oct;170:104578. doi: 10.1016/j.antiviral.2019.104578. Epub 2019 Aug 5. Antiviral Res. 2019. PMID: 31394119
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
-
Zika Virus Vaccine Development: Progress in the Face of New Challenges.Annu Rev Med. 2019 Jan 27;70:121-135. doi: 10.1146/annurev-med-040717-051127. Epub 2018 Nov 2. Annu Rev Med. 2019. PMID: 30388054 Review.
Cited by
-
Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors.Viruses. 2019 Nov 2;11(11):1019. doi: 10.3390/v11111019. Viruses. 2019. PMID: 31684080 Free PMC article.
-
Rapid microsphere-assisted peptide screening (MAPS) of promiscuous MHCII-binding peptides in Zika virus envelope protein.AIChE J. 2020 Mar;66(3):e16697. doi: 10.1002/aic.16697. Epub 2019 Jun 11. AIChE J. 2020. PMID: 33343002 Free PMC article.
-
Multiplex Serology for Sensitive and Specific Flavivirus IgG Detection: Addition of Envelope Protein Domain III to NS1 Increases Sensitivity for Tick-Borne Encephalitis Virus IgG Detection.Viruses. 2024 Feb 13;16(2):286. doi: 10.3390/v16020286. Viruses. 2024. PMID: 38400061 Free PMC article.
-
A 'Furry-Tale' of Zika Virus Infection: What Have We Learned from Animal Models?Viruses. 2019 Jan 6;11(1):29. doi: 10.3390/v11010029. Viruses. 2019. PMID: 30621317 Free PMC article. Review.
-
Neurovirulence of Zika virus-encoded proteins.Arch Virol. 2025 Jun 7;170(7):150. doi: 10.1007/s00705-025-06338-x. Arch Virol. 2025. PMID: 40483309 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
