Genistein Ameliorates Cyclophosphamide - Induced Hepatotoxicity by Modulation of Oxidative Stress and Inflammatory Mediators
- PMID: 29362606
- PMCID: PMC5771282
- DOI: 10.3889/oamjms.2017.093
Genistein Ameliorates Cyclophosphamide - Induced Hepatotoxicity by Modulation of Oxidative Stress and Inflammatory Mediators
Abstract
Aim: The present study investigated the protective effect of the phytoestrogen, genistein (GEN), against (CP)-induced acute hepatotoxicity in rats.
Material and methods: Male adult rats were randomly assigned into five groups. Normal control group received the vehicles; CP group received a single dose of CP (200 mg/kg, i.p). The other three groups received subcutaneous GEN at doses of 0.5, 1 and 2 mg/kg/day, respectively, for 15 consecutive days prior CP injection. Sera and liver tissues were collected forty-eight hours after CP injection for assessment of liver function enzymes (ALT and AST) in rat sera, the hepatic oxidative/nitrosative biomarkers (GSH, MDA and NOx), hepatic interleukin-1β, and myeloperoxidase activity. Immunohistochemistry of cyclooxygenase-2 and histopathological examination of liver tissues were also conducted.
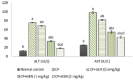
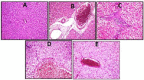
Results: The CP-induced acute liver damage was evidenced by elevated serum ALT and AST accompanied by increased hepatic oxidative stress and inflammatory biomarkers. Immunohistochemical outcomes revealed hepatic cyclooxygenase-2 expression in CP group with distortion of liver architecture. GEN-pretreatment significantly ameliorated the deterioration of liver function and exerted significant anti-oxidant and anti-inflammatory activity with a marked decline in hepatic cyclooxygenase-2 expression in a dose dependent-manner.
Conclusion: The present study demonstrated that the antioxidant and anti-inflammatory activities of GEN might contribute to its protective effects against CP-induced liver damage.
Keywords: Cyclophosphamide; Myeloperoxidase; Oxidative stress; genistein; interleukin-1β.
Figures

References
-
- Moignet A, Hasanali Z, Zambello R, Pavan L, Bareau B, Tournilhac O, Roussel M, Fest T, Awwad A, Baab K, Semenzato G, Houot R, Loughran TP, Lamy T. Cyclophosphamide as a first-line therapy in LGL leukemia. Leukemia. 2014;28:1134–1136. https://doi.org/10.1038/leu.2013.359 PMid:24280867 PMCid:PMC4017255. - PMC - PubMed
-
- Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clinical & translational oncology. 2008;10:583–586. https://doi.org/10.1007/s12094-008-0254-7. - PubMed
-
- Kim SH, Lee IC, Ko JW, Moon C, Kim SH, Shin IS, Seo YW, Kim HC, Kim JC. Diallyl Disulfide Prevents Cyclophosphamide-Induced Hemorrhagic Cystitis in Rats through the Inhibition of Oxidative Damage, MAPKs, and NF-kappaB Pathways. Biomolecules & therapeutics. 2015;23:180–188. https://doi.org/10.4062/biomolther.2014.126 PMid:25767687 PMCid:PMC4354320. - PMC - PubMed
-
- Cuce G, Cetinkaya S, Koc T, Esen HH, Limandal C, Balci T, Kalkan S, Akoz M. Chemoprotective effect of vitamin E in cyclophosphamide-induced hepatotoxicity in rats. Chemico-biological interactions. 2015;232:7–11. https://doi.org/10.1016/j.cbi.2015.02.016 PMid:25779342. - PubMed
-
- Sinanoglu O, Yener AN, Ekici S, Midi A, Aksungar FB. The protective effects of spirulina in cyclophosphamide induced nephrotoxicity and urotoxicity in rats. Urology. 2012;80(6):1392.e1–6. https://doi.org/10.1016/j.urology.2012.06.053 PMid:22951000. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
