Paeoniflorin, the Main Active Ingredient of Shuyu Capsule, Inhibits Cav1.2 and Regulates Calmodulin/Calmodulin-Dependent Protein Kinase II Signalling
- PMID: 29362718
- PMCID: PMC5736929
- DOI: 10.1155/2017/8459287
Paeoniflorin, the Main Active Ingredient of Shuyu Capsule, Inhibits Cav1.2 and Regulates Calmodulin/Calmodulin-Dependent Protein Kinase II Signalling
Abstract
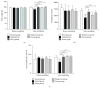
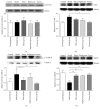
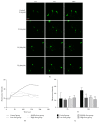
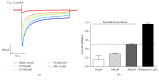
The aim of this study was to explore the mechanism underlying the antidepression activity of paeoniflorin, the main active ingredient of paeony extract and Shuyu capsules, and determine its effect on the calmodulin/calmodulin-dependent protein kinase II (CaM/CaMKII) signalling pathway and on the possible target, the voltage-gated calcium channel (Cav). Rats at the nonacceptance stage were selected for premenstrual syndrome (PMS) depression modelling. Behavioural assays were used for model testing. Rats were given Shuyu capsules, paeony extract, and bupleurum. Western blot analysis was used to assess the expression levels of calcium voltage-gated channel subunit alpha 1 C (CACNA1C), brain-derived neurotrophic factor, and CaM/CaMKII signalling pathway proteins. Intracellular Ca2+ concentration in CHO cell line was measured using Fluo-4-AM and whole-cell patch clamps. The PMS depression model was successfully established and demonstrated that Shuyu can mitigate depressive behaviour in a rat PMS model. Paeony extract did not affect CACNA1C protein expression in rat hippocampi but did affect Cav1.2-mediated CaM/CaMKII signalling pathways. Paeoniflorin significantly inhibited KCl-induced increases in intracellular Ca2+ concentration and Cav1.2 current density. Further, it may function via the CaM/CaMKII pathway and its downstream signalling molecules by regulating Cav1.2, thus playing an important role in the treatment and alleviation of affective disorders.
Figures
Similar articles
-
Abnormal alterations in the Ca²⁺/CaV1.2/calmodulin/caMKII signaling pathway in a tremor rat model and in cultured hippocampal neurons exposed to Mg²⁺-free solution.Mol Med Rep. 2015 Nov;12(5):6663-71. doi: 10.3892/mmr.2015.4227. Epub 2015 Aug 18. Mol Med Rep. 2015. PMID: 26299765 Free PMC article.
-
Paeoniflorin Attenuates Cerebral Ischemia-Induced Injury by Regulating Ca2+/CaMKII/CREB Signaling Pathway.Molecules. 2017 Feb 27;22(3):359. doi: 10.3390/molecules22030359. Molecules. 2017. PMID: 28264448 Free PMC article.
-
CaM-dependent modulation of human CaV1.3 whole-cell and single-channel currents by C-terminal CaMKII phosphorylation site S1475.J Physiol. 2024 Aug;602(16):3955-3973. doi: 10.1113/JP284972. Epub 2024 Jul 22. J Physiol. 2024. PMID: 39037941
-
Regulation of Cardiac Cav1.2 Channels by Calmodulin.Int J Mol Sci. 2023 Mar 29;24(7):6409. doi: 10.3390/ijms24076409. Int J Mol Sci. 2023. PMID: 37047381 Free PMC article. Review.
-
Exploring the dominant role of Cav1 channels in signalling to the nucleus.Biosci Rep. 2012 Dec 20;33(1):97-101. doi: 10.1042/BSR20120099. Biosci Rep. 2012. PMID: 23088728 Free PMC article. Review.
Cited by
-
Gene Expression in the Hippocampus in a Rat Model of Premenstrual Dysphoric Disorder After Treatment With Baixiangdan Capsules.Front Psychol. 2018 Nov 13;9:2065. doi: 10.3389/fpsyg.2018.02065. eCollection 2018. Front Psychol. 2018. PMID: 30483168 Free PMC article.
-
Natural L-type calcium channels antagonists from Chinese medicine.Chin Med. 2024 May 21;19(1):72. doi: 10.1186/s13020-024-00944-8. Chin Med. 2024. PMID: 38773596 Free PMC article.
-
Mechanisms of paeoniaceae action as an antidepressant.Front Pharmacol. 2023 Feb 8;13:934199. doi: 10.3389/fphar.2022.934199. eCollection 2022. Front Pharmacol. 2023. PMID: 36844911 Free PMC article. Review.
-
Allopregnanolone-mediated GABAA-Rα4 function in amygdala and hippocampus of PMDD liver qi-invasion syndrome model rats.Aging (Albany NY). 2023 Feb 23;15(4):1143-1157. doi: 10.18632/aging.204541. Epub 2023 Feb 23. Aging (Albany NY). 2023. PMID: 36842096 Free PMC article.
-
Animal, Herb, and Microbial Toxins for Structural and Pharmacological Study of Acid-Sensing Ion Channels.Front Pharmacol. 2020 Jul 8;11:991. doi: 10.3389/fphar.2020.00991. eCollection 2020. Front Pharmacol. 2020. PMID: 32733241 Free PMC article. Review.
References
-
- Hylan T. R., Sundell K., Judge R. The impact of premenstrual symptomatology on functioning and treatment-seeking behavior: experience from the United States, United Kingdom, and France. Journal of Women's Health & Gender-Based Medicine. 1999;8(8):1043–1052. doi: 10.1089/jwh.1.1999.8.1043. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

